Figure 4.
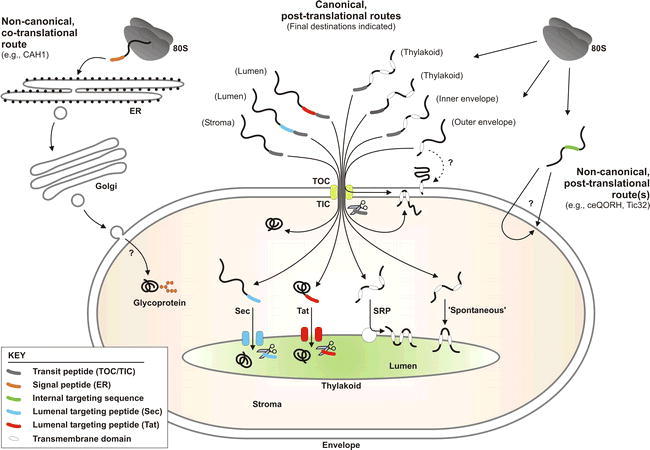
Overview of the protein import and routing systems of chloroplasts.
Most proteins access the chloroplast interior via the TOC/TIC machinery (yellow; centre of figure). Examples of proteins that utilize this canonical pathway are shown schematically, and their final destinations are indicated parenthetically. Transit peptides (see key) mediate envelope translocation, and are cleaved by SPP (represented by scissors) on arrival in the stroma. Then, imported proteins may either adopt their final conformation, or engage one of several internal sorting pathways. Lumenal proteins cross the thylakoid membrane via the Sec pathway (blue) or the Tat pathway (red). Distinct Sec and Tat lumenal targeting peptides engage the respective translocation machineries, and are cleaved by the thylakoidal processing peptidase (TPP; represented by scissors) in the lumen. Most thylakoid membrane proteins do not possess a cleavable targeting signal. Some of these proteins are targeted by the SRP machinery (white), whereas others insert ‘spontaneously’ into the membrane. Similarly, most outer envelope membrane proteins are targeted without the aid of a cleavable targeting signal; while it has been proposed that their insertion occurs spontaneously (see dotted line), recent evidence suggests that such proteins utilize TOC component(s) during their insertion. Two different TOC/TIC-based pathways mediate targeting to the inner envelope membrane: in the ‘post-import’ pathway, complete translocation into the stroma is followed by export to the inner membrane; in the ‘stop-transfer’ pathway, transmembrane domains within the mature part of the protein cause lateral exit from the TIC machinery. Recently, non-canonical, TOC/TIC-independent pathways for chloroplast protein targeting have been identified. In the first of these (right side of figure), proteins with non-cleavable, internal targeting signals are directed to the inner membrane by one or more novel pathways. Such import is energy dependent, but the translocon component(s) have not been identified. In the second (left side of figure), proteins are synthesized with a signal peptide for ER translocation. These proteins follow a pathway through the ER and Golgi, where they may become glycosylated; exactly how such proteins traverse the envelope membranes is not known. This figure has been adapted from Jarvis (2008).
