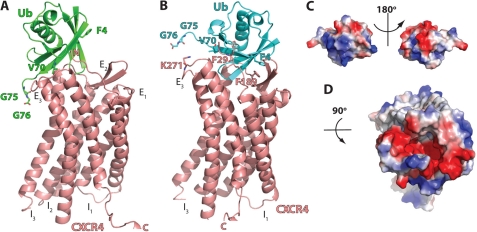
FIGURE 3.
Protein-protein docking of ubiquitin onto the extracellular domain of CXCR4. A, ribbon diagram of the ubiqutin-CXCR4 complex from the lowest energy model of Rosetta docking. Ub and CXCR4 are colored in green and salmon, respectively. The N terminus (N), extracellular loops (E1–E3), intracellular loops (I1–I3), and C terminus (C) of CXCR4 and ubiquitin residues that were identified to be important for CXCR4 binding and activation are indicated. B, ribbon diagram of the ubiquitin-CXCR4 complex after manual adjustment of the docking based on the charge complementarity and the mutational data. Ub and CXCR4 are colored in cyan and salmon, respectively. The ubiquitin and CXCR4 residues that are predicted as interaction sites are indicated. C, surface representation of ubiquitin. The orientation of ubiquitin is the same as in B. D, surface representation of the extracellular domain of CXCR4.