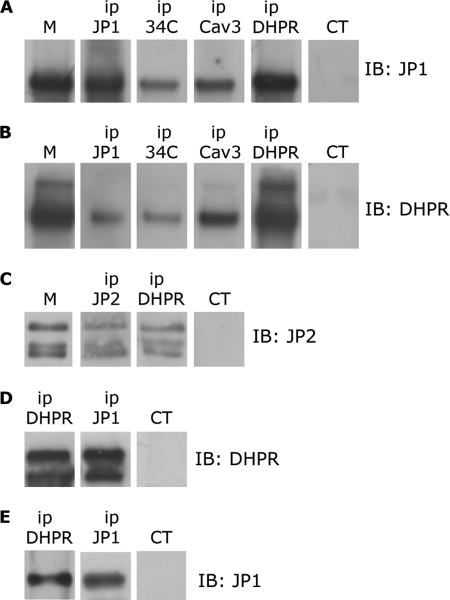
FIGURE 1.
Immunoprecipitation of DHPR, JP1, and JP2 from solubilized rabbit skeletal muscle and HEK293-T microsomes. A, 100 μg of proteins solubilized from rabbit skeletal muscle microsomes (M) were immunoprecipitated (ip) with antibodies against JP1, RyR (34C), caveolin-3 (Cav3), α1s-DHPR (DHPR), and a non-correlated control antibody (CT). After gel electrophoresis, proteins were transferred to a nylon membrane and incubated with anti-JP1 antibody. B, 100 μg of proteins solubilized from skeletal muscle microsomes were immunoprecipitated with antibodies against JP1, RyR, caveolin-3, and α1s-DHPR and a non-correlated control antibody. After gel electrophoresis, proteins were transferred to a nylon membrane and incubated with an antibody against α1s-DHPR. C, 100 μg of proteins solubilized from rabbit skeletal muscle microsomes were immunoprecipitated with antibodies against JP2, α1s-DHPR, and a non-correlated control antibody. After gel electrophoresis, proteins were transferred to a nylon membrane and incubated with anti-JP2 antibody. D, solubilized proteins from HEK293-T cells transfected with pEGFP-α1s-DHPR, pEGFP-β1a-DHPR, and pCDNA-JP1 were immunoprecipitated with antibodies against α1s-DHPR, JP1, and a non-correlated control antibody. Proteins were separated by gel electrophoresis, transferred to a nylon membrane, and incubated with an antibody against DHPR. E, solubilized proteins from HEK293-T cells transfected with pEGFP-α1s-DHPR, pEGFP-β1a-DHPR, and pCDNA-JP1 were immunoprecipitated with antibodies against α1s-DHPR, JP1, and a non-correlated control antibody. Proteins were separated by gel electrophoresis, transferred to a nylon membrane, and incubated with an anti-JP1 antibody.