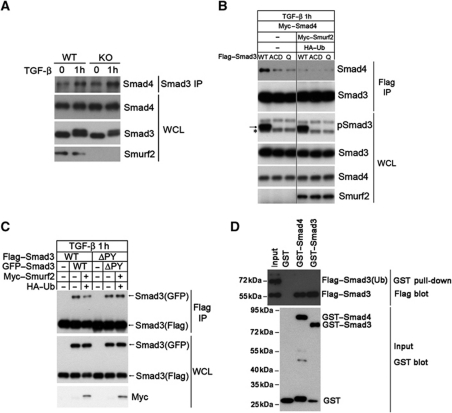
Figure 7.
Mono-ubiquitination of Smad3 destabilizes Smad complexes. (A) Loss of Smurf2 enhances the formation of the endogenous Smad3/Smad4 heteromeric complex. MEFs were treated±TGF-β for 1 h before subjecting to IP-western blot analysis. (B) Reintroducing Smurf2 into Smurf2−/− MEFs disrupts the exogenous Smad3/Smad4 complex. The asterisk denotes the endogenous phosphorylated Smad3, and the arrow denotes the exogenous phosphorylated Smad3. Note that the triple and quadruple mutants are defective in receptor activation and Smad4 interactions. (C) Reintroducing Smurf2 back to Smurf2−/− MEFs reduces formation of the exogenous Smad3 homomeric complex. Note the changes in the amount of Smad3(GFP) brought down by anti-Flag IP in the absence or presence of Smurf2 and HA–Ub or with the ΔPY mutant. (D) GST pull-down assay showing that mono-ubiquitinated Smad3 does not bind the purified Smad4 or phospho-Smad3. The 1:1 mixture of ubiquitinated and non-ubiquitinated Smad3SD was prepared from transfected HEK293 cells, and incubated with GST, GST–Smad4, or phosphorylated GST–Smad3, respectively, before being processed for GST pull-down and western blot analysis. Figure source data can be found with the Supplementary Information.