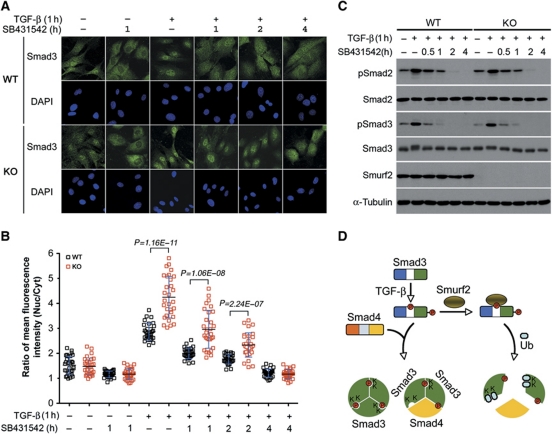
Figure 8.
Loss of Smurf2 increases nuclear accumulation of Smad3. (A) Indirect immunofluorescence staining of endogenous Smad3. MEFs were pretreated±TGF-β for 1 h, followed by ligand removal and receptor blocking with SB431542 for indicated duration. (B) Quantification of fluorescence intensity in (A). The results are expressed as ratios of mean fluorescence intensity between nuclear and cytoplasmic staining. Bars indicate mean±s.d., and n=30 in each condition. (C) Western blot analysis of phospho-Smad2/3 showing similar kinetics of dephosphorylation between WT and KO MEFs. TGF-β and SB431542 treatments were as in (A). (D) A model for the inhibitory function of Smurf2 in TGF-β signalling via mono-ubiquitination of Smad3. Upon TGF-β stimulation, Smad3 is phosphorylated at sites in both the linker and the C-terminal regions. Phosphorylation of T179 in the linker region potentiates Smurf2 binding and subsequent mono-ubiquitination in the MH2 domain. This mono-ubiquitination impedes formation of both heterotrimeric and homotrimeric Smad3 complexes, thereby restraining the amplitude of TGF-β signalling. Figure source data can be found with the Supplementary Information.