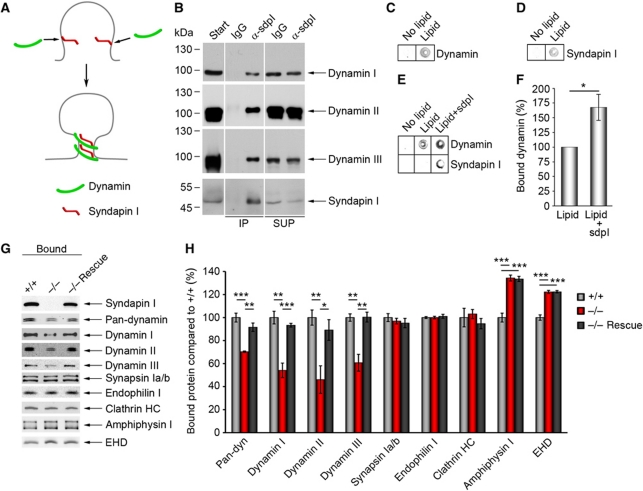
Figure 6.
Syndapin I promotes lipid association of all dynamins and is of critical importance for dynamin membrane anchoring. (A) Scenario, in which syndapin I coordinates the vesicle formation machinery with membrane curvature by direct binding to curved lipid surfaces and by dynamin recruitment. (B) Specific detection of dynamins I, II and III in anti-syndapin I immunoprecipitates from brain extracts (IPs). SUP, supernatant. The order of samples on the gels was modified, as indicated by white lines. (C–F) Lipid-binding assay in 96-well plates coated with or without phosphatidylserine (PS) and phosphatidylcholine (PC) mixture (PS:PC ratio 1:3) revealed a specific lipid binding of dynamin (C) and syndapin I (D). (E) Preincubating lipid-coated wells with syndapin I strongly increased subsequent binding of dynamin. Quantitative analyses (F) show a significant increase (167±22%) (Wilcoxon signed rank test). Data represent mean±s.e.m. N=6 experiments; *P<0.05. (G, H) Liposomes made of Folch fraction I incubated with equal amounts of WT and syndapin I KO cytosol and analysed by quantitative western blot analysis (H). Note the significant reduction of dynamin binding (30% for pan-dynamin, 40% for dynamins I and III and 50% for dynamin II), whereas synapsin Ia/b, endophilin I and clathrin heavy chain (HC) binding were unaffected. The dynamin recruitment defects in syndapin I KO cytosol were completely abolished by addition of syndapin I (−/− Rescue) (G, H). Data represent mean±s.e.m. of four independent experiments with material from 2 animals/genotype. One-way ANOVA; *P<0.05, **P<0.01, ***P<0.001. Figure source data can be found in Supplementary data.