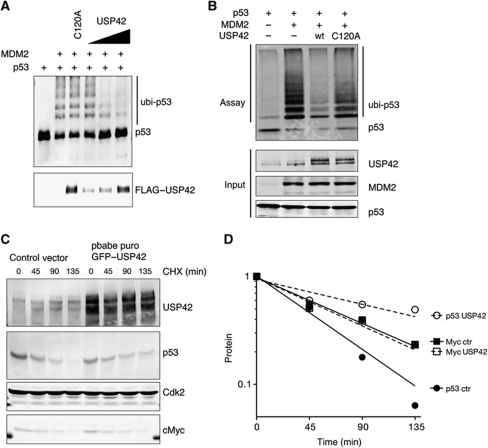
Figure 3.
USP42 deubiquitininates p53. (A) In vitro translated p53 was pre-incubated for 90 min with a bacterially expressed MDM2 and subsequently immunoprecipitated with an anti-p53 antibody. Immunoprecipitated p53 was then incubated with in vitro translated wild-type (wt) or C120A Flag-tagged USP42, followed by western blotting with anti-p53 and anti-USP42. (B) U2OS cells were co-transfected with p53, MDM2, wild-type or C120A GFP–Flag–USP42 and HIS–ubiquitin, as indicated. Twenty-four hours after transfection, lysates were precipitated with HIS-tag isolation Dynabeads and western blotted for p53. The input level of each protein was assessed by western blotting (below). (C) U2OS cells were infected with wild-type GFP–Flag-tagged USP42 or control vector. Seventy-two hours after transfection, cells were selected with puromycin for 2 weeks to establish USP42 overexpressing or control cell pools. These cells were treated for the indicated time with 100 μg/ml of cyclohexamide to inhibit the translation. Cell lysates were prepared at these times and probed for USP42, p53 and cMyc protein level by western blot. Cdk2 was used as loading control. (D) Quantification of (C). Figure source data can be found with the Supplementary data.