Abstract
Study Objective
To review reasons for suboptimal recruitment for a randomized controlled trial (RCT) of varicocelectomy vs. intrauterine insemination for treatment of male infertility, and suggest means to improve future study recruitment.
Design
A survey of RMN participating sites.
Setting
The Reproductive Medicine Network.
Patients
N/A
Interventions
N/A
Main Outcome Measures
Ascertain reasons for inadequate recruitment and suggest improvements for future varicocelectomy trails.
Results
This study screened 7 and enrolled 3 couples with the first couple randomized on 6/30/2010. The study was subsequently stopped on 03/30/2011. The following themes were cited most frequently by sites and therefore determined to be most likely to have played a role in suboptimal recruitment: (1) men must be screened at the beginning of a couple's infertility evaluation, (2) inclusion of infertile women who have failed previous fertility interventions appeared to be associated with couple intolerance of a placebo arm, and (3) there appeared to be bias against the use of unstimulated IUI cycles, indicating a prejudicial preference for surgical intervention in the male partner.
Conclusions
Improved recruitment may be realized through screening infertile men as early as possible while minimizing study-related time commitments. Focused patient education may promote improved ‘equipoise’ and acceptance of a placebo arm in male infertility studies. Lastly, creative approaches to implementing varicocelectomy trials must be considered in addition to having a network of motivated researchers who carry a high volume of possible study participants, as screening of very large numbers may be needed to complete clinical trial enrollment. Clinicaltrials.gov: NCT00767338.
Keywords: Recruitment, consent, randomization, accrual, enrollment, prospective, varicocele, varicocelectomy
Introduction
The Reproductive Medicine Network (RMN) is a multicenter clinical trial network funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) to formulate and conduct clinical trials focused on improving reproductive outcomes in subfertile populations. The current iteration of the RMN is unique in that it included co-investigators from each RMN unit (RMNU), with expertise in male-factor infertility, giving them a mandate to design and implement a clinical trial specifically focused on male infertility. Two male-factor projects were developed. The first project was not implemented due to concern that an adequate numbers of subjects could not be enrolled. The second project, a varicocele trial titled “A Prospective, Randomized Study of Microsurgical Varicocelectomy versus No Surgery in the Treatment of Male Partners with a Palpable Varicocele and an Abnormal Semen Analysis” was prioritized to begin. Unexpected poor recruitment led to closure by the Data Safety Monitoring Board (DSMB). In this article we critically review the investigators' collective experience from all 5 participating sites in an effort to identify reasons for poor recruitment and develop methods to overcome them.
Materials and Methods
We critically reviewed the experiences of each RMN site to assess subject recruitment. This feedback was obtained through teleconferences, face to face meetings, questionnaires emailed to each participating site, and review of data submitted to the Data Coordinating Center (DCC). Upon cancellation, further feedback and pertinent information was collected from the co-investigators, gathered into themes, and summarized.
Study Overview
The primary goal of the study was to determine whether varicocelectomy increased the rate of pregnancy and live birth compared with intrauterine insemination (IUI) or timed intercourse. Inclusion criteria for the varicocelectomy trial were at least 6 months of infertility as a couple and the ability to have regular intercourse. Males between 18 and 50 years of age with bilateral grade I or unilateral grade II-III varicoceles on physical examination and having an abnormal semen analysis were eligible to participate. An abnormal semen analysis was defined as isolated oligospermia (a sperm concentration of between 5 and 20 million/cc) or, a sperm concentration of at least 5 million/cc with sperm motility less than 50% (using WHO II criteria) or a strict morphology score of less than 15% (as defined by Kruger criteria). Their female partners were between 18 and 40 years of age with evidence of at least one patent fallopian tube, a history of regular ovulatory cycles (lasting 25-35 days) with the ability to detect a mid-cycle urinary LH surge using an ovulation predictor kit.
Exclusion criteria included previous sterilization procedures such as vasectomy or tubal ligation. Males were excluded for recurrent varicocele, the presence of retrograde ejaculation, or uncorrectable ejaculatory dysfunction. Female partners were excluded for decreased ovarian reserve as evidenced by a day 3 FSH ≥ 12 mIU/ml, current pregnancy, or medical contraindications to pregnancy as determined by each site investigator's local clinical practice.
Figure 1 summarizes the study design. The project involved two separate randomizations: (1) eligible males were randomized to microsurgical varicocelectomy or no surgery; (2) eligible couples were randomized to start conception attempts with either timed intercourse or un-stimulated intrauterine insemination (IUI). Future cycles for each couple would then alternate between timed intercourse and IUI until either a total of 8 cycles had transpired, or conception occurred. The unique aspect of this study design is that the placebo arm for surgical therapy included medical treatment with IUI alternating with timed intercourse. The rationale for performing unstimulated IUI was to show as a secondary outcome, the added value of IUI over intercourse in women with normal cycles. Most of the published literature showing the potential benefit of varicocelectomy versus no surgery has focused on the incidence of pregnancy over time in couples where the female partner is not receiving ovulation induction treatment. Although it is often common clinical practice to offer controlled ovarian stimulation with IUI as a therapy for couples with varicocele associated infertility; incorporating this methodology into the study posed difficulty with statistical modeling to achieve sufficient power without the framework of published reference studies. We followed the methodology of prior studies (with the exception of adding unstimulated IUI) since the effectiveness of ovulation induction with IUI has never been evaluated in comparison to simple observation in this population. Noting the difficulties in recruitment with our study design suggests a need for additional clinical research on the relative effectiveness of treatment options for varicoceles other than surgery.
Figure 1. Varicocelectomy Flow Chart.
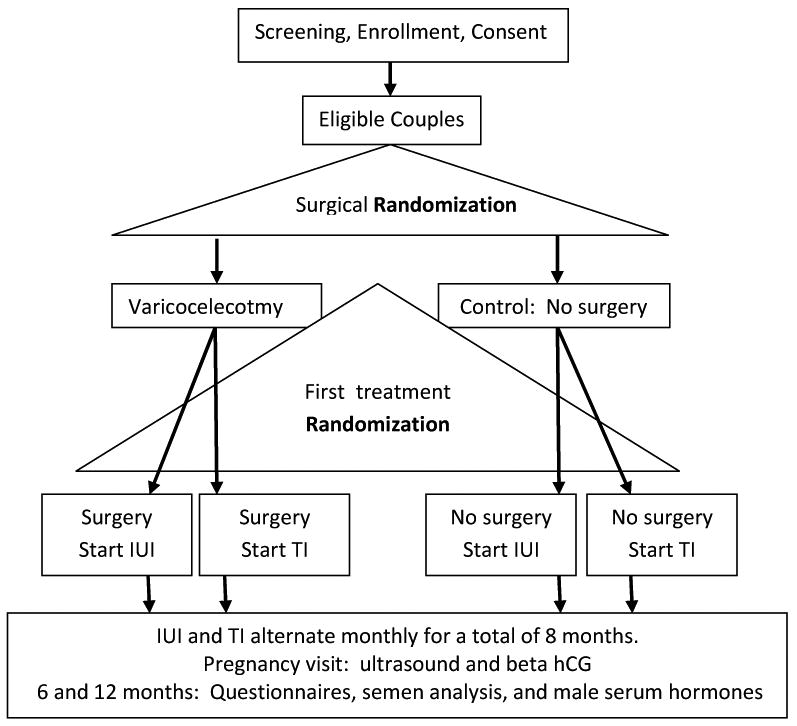
After eight months of alternating timed intercourse (4) with IUI (4) cycles, the couples were to continue monthly contact with their research coordinators to track pregnancy rates and secondary outcome measures for a total of 12 months.
Power Calculations for the original RMN varicocele trial
The proposed study was powered (using nQuery Advisor 6.01) to test if there would be a difference in pregnancy rates between the two main arms of the study (varicocelectomy vs. no varicocelectomy) as well as the effects of timed intercourse vs. IUI. Reviews from several other studies found a pregnancy rate (over 1 year) of 33 to 36.4 percent in couples after varicocelectomy, as compared with 16 to 20 percent in untreated couples (1, 2). Similar pregnancy rates (32.9 and 13.9 percent at one year) were reported (subsequent to this power analysis) by Abdel-Meguid (3). A study with a similar design to the RMN trial, investigated whether varicocele treatment before IUI significantly affected IUI success rates (4). In that study, a total of 58 infertile couples, were included in the study and the pregnancy rate was significantly higher in the treated group (32.4%) when compared to the untreated group (16.7%) well within the range previously observed (1,2).
The requisite sample size was set to maintain 80% power with the significance level at 0.05 for a two-sided test. We examined a variety of detectable effects sizes when we fixed 50 patients in each of the four treatment groups. For convenience, we calculated the detectable differences by considering two of the treatment groups (e.g. varicocelectomy followed by IUI first vs. varicocelectomy followed by time intercourse first) at a time. With this design, we could detect an absolute difference of approximately 25% (41% vs. 16%), which was somewhat higher than, but within a reasonable range of what has been previously reported. Our study power is greater than 80% if we merge four arms into two major arms each with 100 patients. The total required sample size is 200. To account for the possibility of up to 20% study dropout in both groups, the goal was to enroll 232 couples from all RMN clinical sites. This would require recruitment of about 33 couples from each of the 7 (original) clinical sites of the RMN.
Study-related Feedback
The RMN investigators who provided patient recruitment-related feedback included a spectrum of male reproductive specialists consisting of 3 urologists, 4 reproductive endocrinologists (REI's), a reproductive geneticist, and a male reproductive endocrinologist.
The following factors were suggested by the investigators as possible impediments to recruitment and thus, the ability to complete the study.
Limited number of sites
Initially, all 7 of the RMN sites were expected to participate in the trial. From this group, 5 sites obtained IRB approvals and were available to recruit for this study. During this period, 3 sites had a RMN Co-investigator (microsurgeon) on staff, and 2 sites lost their urology support (moved to a different academic center or clinical practice location). There was an effort to expand the pool of surgeon investigators to high-volume centers, which yielded 2 additional surgeons. One site noted limited interest of microsurgeons in this study and, therefore, did not recruit.
Bias
Some investigators or referring providers may have unwittingly communicated their personal belief that an IUI or a varicocelectomy is clearly the better option. To these, a randomized trial is not viewed as important or even necessary, so they may chose to not refer patients who would otherwise qualify. In addition, reproductive endocrinologists may view that stimulated IUI cycles are the standard of care and that an un-stimulated IUI cycle is not a wise option. Moreover, potential subjects tended to be older and were not typically treatment-naive; thus, they were perceived as having a low tolerance if they were randomized to the no varicocelectomy arm that would involve up to 4 months of no specific treatment. Many couples visit their gynecologist with fertility concerns and the female partners are started on clomiphene citrate as an initial intervention. Many REI's stated that they tend to see couples after the female partner has failed medical management; thus, REIs may not constitute the best referral source for the trial.
In the era of IUI/IVF, many couples prefer the option of “aggressive” treatment. Therefore, the lack of ovulation stimulation in the IUI arm would appear less attractive to those considering participation, especially if they were to wind up randomized to the non-surgical arm. Moreover, many potentially eligible couples had already tried stimulated IUI without success.
Many female partners had other reproductive issues (in addition to their male partner having a varicocele). For these, an un-stimulated IUI cycle may not be an adequate inducement no matter what other factors are involved. Evaluation of the male partner beyond a semen analysis is often not a part of the couple's initial infertility analysis. A couple whose only identifiable issue is a male varicocele tends to be a small subset of infertile couples, limiting the pool of eligible couples. The male is an equal partner in reproduction and should undergo as timely and complete a screening as the female. At the point of considering enrolling in a fertility study, men tended to push for intervention. Specifically, they desired surgical intervention sooner rather than later. Moreover, they tended to see the existing interval of infertility as their “placebo arm” and wanted to proceed with an active intervention.
Discussion
Increasing evidence suggests that varicocele ligation improves semen quality and pregnancy rates in couples with infertility. Unfortunately, the majority of these data come from retrospective, poorly controlled studies. The Male Infertility Best Practice Policy Committee of the American Urological Association and the Practice Committee of the American Society for Reproductive Medicine has recommended, based on the available literature that varicocele surgery should be offered to the male partner of a couple attempting to conceive, when all of the following are present: 1) a varicocele is palpable 2) the couple has documented infertility 3) the female partner has normal fertility or potentially correctable infertility and 4) the male partner has one or more abnormal semen parameters or sperm function test results (5).
A RCT provides level-I evidence, as well as the most unbiased (6) and reliable evidence for evaluating the effect of a health care intervention (7-8). However, recruiting clinicians and patients to randomized trials can be extremely difficult, (9) which can result in a study being underpowered, over budget, and/or incomplete (10-11). An underpowered study may inadvertently report that a clinically relevant effect does not have statistical significance. A non-significant finding could delay or eliminate consideration of the use of an effective intervention (9). The absence of evidence that a difference exists does not mean that a difference would not be realized if the sample size were larger (12). With increasing reliance on RCT findings for clinical and regulatory decisions, (13) the importance of a successful, accurate, and properly powered trial, with prompt and adequate recruitment cannot be understated.
Historically, recruitment for RCT's would use “trial and error” (14) whereby a study would start by utilizing multiple recruitment techniques, and then, the more successful recruitment method(s) would be targeted for future effort. It is estimated that less than 50% of the studies were able to meet their targeted enrollment, and a minority were able to meet enrollment numbers without extending the trial's time line (15-18). In addition, McDonald reported that the overall start to recruitment was delayed in 41% (n = 47) of trials, while early recruitment problems were identified in 63% (n = 77) of trials (18). Specific to varicoceles, a number of systematic reviews have concluded that properly conducted RCTs on the topic are scarce and often contradictory (2-3, 19-22).
The RMN's varicocelectomy study was posted on Clinicaltrails.gov on October 02, 2008, and subsequently stopped on March 30, 2011. The interval required for IRB review and approval ranged from 2 to 3.5 months, which is similar to the time required for other studies in this same (RMN) network. The majority of the time was spent resolving unique challenges for this trial before the protocol was submitted for IRB review. A visual timeline for the study is provided on figure 2. There were 5 sites available; two sites screened 7 couples of which 3 were randomized. The first patient was randomized on 06/03/2010. The second site enrolled their sole patient on 12/01/2010. The study was stopped on 03/30/2011 due to the likelihood that the required number of participants could not be recruited over the projected remaining time period.
Figure 2. RMN Recruitment Flowchart.
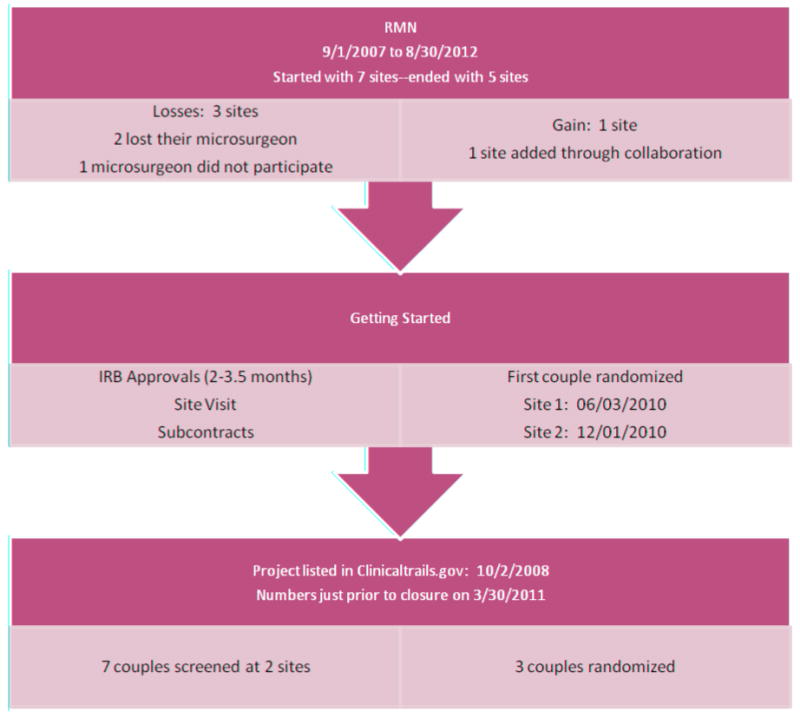
Unlike three other prospective studies underway with the current iteration of the RMN where recruitment (over 1200 subjects so far) has proceeded according to expectations, the RMN's RCT on microscopic varicocelectomy experienced not only a delayed startup, but substantial difficulties with recruitment, which resulted in discontinuation. Upon review, it became apparent that the several factors played a role in this outcome; some are common to many RCTs and others are more specific to the trial. (1) delayed start-up for such an RCT is particularly harmful, as it reduces available recruitment time, (2) when screening for male-factor infertility occurs late in the couples' evaluation, it becomes more likely that other infertility factors will be found, rendering the couple ineligible or intolerant of male-only interventions and (3) infertile women screened for this trial had often failed previous interventions and given the option, their male partners disliked entering an “inactive placebo” arm. Instead, many men wanted to proceed directly with surgery.
Review of the Literature Regarding Recruitment
There have been several studies and reviews examining specific ways to improve recruitment into a randomized controlled trial (RCT) (9, 13, 23). Based on varicocelectomy literature, review articles, meta-analyses, and a Cochrane review, recurring critiques stand out as to why this body of literature is so difficult to assimilate. Enrollment for the most recent varicocelectomy RCT's are listed in Table 1. There is lack of consensus among reviewers with some reviews espousing varicocelectomy benefits while others refuting this conclusion (24-25). There is thus no consensus on what constitutes the best practice and we continue to believe that a randomized, clinical trial is necessary to answer this question in a definitive fashion.
Table 1. Recent Prospective Randomized Varicocelectomy Trials.
| Prospective Study | Screened | Chose Assisted Reproduction | Randomized (% enrolled) | Drop out | Intervention/Control |
|---|---|---|---|---|---|
| Nilson 1979 (27) | 165 | N/A | 96 (58%) | N/A | N/A |
| Breznik 1993 (28) | 96 | N/A | 79 (82%) | N/A | 38/41 |
| Madgar 1995 (29) | 57 | N/A | 45 (79%) | N/A | 25/20 |
| Nieschlag 1998 (30) | 226 | 23 (10%) | 203 (90%) | 78 (38%) | 62/63 |
| Yamamoto 1996 (31) | 92 | N/A | 92 (100%) | 7 (8%) | 45/40 |
| Abdel-Meguid 2011 (3) | 251 | N/A | 150 (60%) | 5 (0.03%) | 73/72 |
| Current Trial | 7 | 1 (14%) | 3 (43%) | 1 (33%) | 0/3 |
Not available = N/A
This RMN varicocelectomy trial attempted to proactively address as many controversial points as possible in its design. Table 2 lists specific RMN protocol decisions next to critiques of prior studies.
Table 2. RMN Response to Critiques of other Varicocelectomy Trials.
| Critique | RMN Varicocelectomy Trial |
|---|---|
| With IVF/ICSI, men are reluctant to risk “no treatment arm” | Un-stimulated IUI cycles provide an intervention/treatment |
| Post-op: improved sperm parameters and pregnancy rates may take up to 5 and 7 months respectively. | Captures pregnancies and live births for up to 12 months |
| Sperm density improvements better with initial sperm concentrations greater than 10 million/cc. Some included men with normal sperm parameters | Must have isolated oligospermia (5-20 M/cc) -or-concentration over 5 M/cc AND motility < 50% OR strict Kruger morphology < 15%. |
| Confounding variables such as differing varicocelectomy techniques (embolization, sclerosis, various surgical approaches). | Only surgical option was a microsurgical inguinal/sub-inguinal repair. |
| Included subclinical varicoceles | Bilateral grade I or unilateral grade II-III on physical exam. |
| No assessment of semen analysis to a specific time after surgery | Compares a screening semen analysis to one 12 months post-op |
| No record of randomization technique | A unique identifier yields two computer generated randomizations: (1) surgery or not, and (2) start alternating fertility attempts with IUI or timed intercourse |
| Lacked the couples baseline characteristics including female reproductive capacity | Will record a history and physical, baseline characteristics, evidence for regular periods, a patent fallopian tube, and ovarian reserve (day 3 FSH < 12 mIU/ml) |
| Reported improved semen parameters and fertility rates, not live birth rates | The primary outcome is an improvement in live birth rates |
| Lacked drop-out or lost to follow-up rates | Minimize drop-out rates by offering alternating IUI cycles for the first 8 (of 12) months |
Lastly, a single varicocelectomy RCT reported poor recruitment, a high drop-out rate of greater than 50%, and more importantly, specific reasons for these challenges (26). Krause et al compared pregnancy rates one year after antegrade/retrograde varicocele sclerosis versus “wait and see.” This was a collaborative, multi-institutional study involving 15 German andrology centers whose initial goal was to recruit 300 subjects. Seven reasons were provided by Krause et al to account for their challenges with recruitment and follow-up:
Private physicians had already diagnosed males with a varicocele and referred the males to the andrology center for treatment. Subjects anticipated surgical intervention.
Many patients and doctors prefer the immediate intervention of assisted reproductive techniques (IUI/ICSI) as compared to waiting for slow improvement in sperm parameters (after varicocelectomy).
Patients themselves desired immediate, active treatment targeted to the male partner.
Patients and doctors found it hard to accept treatment based on computer randomization.
The andrology centers overestimated the number of patients presenting with varicoceles.
The medical staff was not sufficiently motivated to recruit patients.
Medical staff turn-over hampered record completeness.
For success, complex RCT's, such as this varicocelectomy study, will require a flexible platform. To accomplish this, a pilot study may be considered that would allow for both an interim endpoint analysis in addition to opportunities to review recruitment challenges and adjust the protocol accordingly. Feedback provided by post-enrollment patient questionnaires or even patient-based focus groups could be used to discern reasons for study declinations or suggestions for study improvement. Moreover, drop-out rates tended to be high in this younger, mobile population and must be accounted for, either by more careful screening or larger sample sizes. Recruitment may be enhanced by offering a “crossover” period whereby those in the control arm can subsequently choose varicocelectomy. Finally, an opportunity for future collaboration and data pooling may be possible. For example, there is a prospective varicocelectomy study from Canada, currently awaiting accrual, looking for post-varicocelectomy improvements in fertility and testicular function (Clinical Trails.gov Identifier NCT00961558).
To optimize any RCT recruitment, patients need to be educated in an unbiased fashion about their particular health problem. The consent process should be easy to understand and culturally sensitive. To optimize varicocelectomy RCT recruitment, male partners of infertile couples should be evaluated as early as possible. Enabling participation of clinical urologists who are interested in taking part in studies that answer important clinical questions regarding surgical treatments would increase the pool of men with varicoceles who could be recruited. Funding for such trials may need to be expanded to insurers and other funding sources interested in identifying cost-effective treatments. This would extend the life span of a slow-recruiting trial beyond that of the period of an NIH-funded clinical trials network (such as the RMN).
Acknowledgments
This work was supported by National Institutes of Health (NIH)/Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Grants U10 HD27049 (to C.C.), U10 HD38992 (to R.S.L.), U10HD055925 (to H.Z. and M.Z.), U10 HD39005 (to M.P.D.), U10 HD38998 (to W.D.S.), U10 HD055936 (to G.M.C.), U10 HD055942 (to R.G.B.), and U10 HD055944 (to P.R.C.)
J C Trussell, M.D. Financial support: National Institutes of Health (NIH)/Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Grant U10 HD38992
Gregory M. Christman, M.D. Financial support: National Institutes of Health (NIH)/Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Grant U10 HD055936.
Dana A. Ohl, M.D. Financial support: National Institutes of Health (NIH). Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Grant U10 HD055936
Richard S. Legro, M.D. Financial support: National Institutes of Health (NIH)/Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Grant U10 HD38992
Stephen A. Krawetz, PhD. Financial support: National Institutes of Health (NIH)/Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Grant U10 HD39005.
Peter J. Snyder, M.D. Financial support: None reported
Pasquale Patrizio, M.D., MBE. Financial support: National Institutes of Health (NIH)/Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Grant U10HD055925
Alex J. Polotsky, M.D. Financial support: Bayer (unrestricted research grant)
Michael P. Diamond, M.D. Financial support: National Institutes of Health (NIH)/Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Grant U10 HD39005
Peter R. Casson, M.D. Financial support: National Institutes of Health (NIH)/Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Grant U10 HD055944
Christos Coutifaris, M.D., PhD. Financial support: National Institutes of Health (NIH)/Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Grant U10 HD27049.
Kurt Barnhart, M.D., MSCE. Financial support: None reported
Robert G. Brzyski, M.D., PhD. Financial support: National Institutes of Health (NIH)/Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Grant U10 HD055942
William D. Schlaff, M.D. Financial support: National Institutes of Health (NIH)/Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Grant U10 HD38998.
Randall Meacham, M.D. Financial support: None reported
David Shin, M.D. Financial support: None reported
Tracey Thomas, MPH. Financial support: National Institutes of Health (NIH)/Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Grant U10HD055925
Meizhuo Zhang, PhD. Financial support: National Institutes of Health (NIH)/Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Grant U10HD055925
Nanette Santoro, M.D. Financial support: None reported
Esther Eisenberg, M.D., MPH. Financial support: None
Heping Zhang, Ph.D. Financial support: National Institutes of Health (NIH)/Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Grant U10HD055925
Footnotes
J C Trussell, M.D. Conflict of interest: None
Gregory M. Christman, M.D. Conflict of interest: None
Dana A. Ohl, M.D. Conflict of interest: None reported
Richard S. Legro, M.D. Conflict of interest: None reported
Stephen A. Krawetz, PhD. Conflict of interest: None
Peter J. Snyder, M.D. Conflict of interest: None reported
Pasquale Patrizio, M.D., MBE. Conflict of interest: None
Alex J. Polotsky, M.D. Conflict of interest: None
Michael P. Diamond, M.D. Conflict of interest: None reported
Peter R. Casson, M.D. Conflict of interest: None reported
Christos Coutifaris, M.D., PhD. Conflict of interest: None reported
Kurt Barnhart, M.D., MSCE. Conflict of interest: None reported
Robert G. Brzyski, M.D., PhD. Conflict of interest: None reported
William D. Schlaff, M.D. Conflict of interest: None reported
Randall Meacham, M.D. Conflict of interest: None reported
David Shin, M.D. Conflict of interest: None reported
Tracey Thomas, MPH. Conflict of interest: None reported
Meizhuo Zhang, PhD. Conflict of interest: None reported
Nanette Santoro, M.D. Conflict of interest: None reported
Esther Eisenberg, M.D., MPH. Conflict of interest: None
Heping Zhang, Ph.D. Conflict of interest: None
For the Reproductive Medicine Network.
Experiences of a varicocelectomy trial stopped for poor recruitment yield suggestions to improve success with future male infertility clinical trials.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
JC Trussell, Associate Professor, Urology. Upstate University Hospital, NY
Gregory M. Christman, Associate Professor, Obstetrics & Gynecology, University of Michigan, MI
Dana A. Ohl, Professor of Urology, Head, Division of Sexual and Reproductive Medicine, University of Michigan, Ann Arbor, MI
Richard S. Legro, Professor, Department of Obstetrics and Gynecology, Pennsylvania State University School of Medicine, PA
Stephen A. Krawetz, Professor of Fetal Therapy and Diagnosis, Department of Obstetrics and Gynecology, Center for Molecular Medicine and Genetics. Wayne State University, MI
Peter J. Snyder, Professor of Medicine. University of Pennsylvania, PA
Pasquale Patrizio, Professor, Department of Obstetrics and Gynecology. Yale University School of Medicine, CT
Alex J. Polotsky, Assistant Professor, Department of Obstetrics and Gynecology. University of Colorado, CO
Michael P. Diamond, Professor; Assistant. Dean of Clinical & Translational Research; Assoc. Chair-Department of Obstetrics and Gynecology. Wayne State University, MI
Peter R. Casson, Professor of Obstetrics and Gynecology. University of Vermont, VT
Christos Coutifaris, Professor of Obstetrics and Gynecology, University of Pennsylvania, PA
Kurt Barnhart, Professor of Obstetrics and Gynecology. University of Pennsylvania, PA
Robert G. Brzyski, Professor of Obstetrics and Gynecology. The University of Texas Health Science Center at San Antonio, TX
William D. Schlaff, Professor of Obstetrics and Gynecology. University of Colorado, CO
Randall Meacham, Professor and Chief of Urology at the University of Colorado, CO
David Shin, Attending, Department of Urology. Hackensack University Medical Center, NJ
Tracey Thomas, Project Manager, Research Associate. Yale University, CT
Meizhuo Zhang, Project Director. Yale University, CT
Nanette Santoro, Professor and Chair, Department of Obstetrics and Gynecology. University of Colorado, CO
Esther Eisenberg, Professor of Obstetrics and Gynecology. Vanderbilt University, TN and Eunice Kennedy Shriver National Institute of Child Health and Human Development, Bethesda, MD
Heping Zhang, Professor, Department of Epidemiology and Public Health. Yale University, CT
References
- 1.Sharlip ID, Jarow J. American Urological Association Practice Committee Report on Varicocele and Infertility. AUA. 2001:1–9. [Google Scholar]
- 2.Ficarra V, Cerruto MA, Ligouri G, Mazzoni G, Minnuci S, Tracia A, et al. Treatment of Varicocele in Subfertile Men: The Cochrane Review—A Contrary Opinion. Eur Urol. 2006;49(2):2580263. doi: 10.1016/j.eururo.2005.11.023. [DOI] [PubMed] [Google Scholar]
- 3.Abdel-Meguid TA, Al-Sayyad A, Tayib A, Farsi HM. Does varicocele repair improve male infertility? An evidence-based perspective from a randomized, controlled trial. Eur Urol. 2011;59:455–461. doi: 10.1016/j.eururo.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 4.Daitch JA, Bedaiwy MA, Pasqualotto EB, Hendin BN, Hallak J, Falcone T, et al. Varicocelectomy improves intrauterine insemination success rates in men with varicocele. J Urol. 2001;165(5):1510–3. [PubMed] [Google Scholar]
- 5.American Urological Association Practice Committee Report on Varicocele and Infertility. Fertil Steril. 2004;82:142–145. [Google Scholar]
- 6.Kunz R, Vist G, Oxman AD. Randomisation to protect against selection bias in healthcare trials. Cochrane Database of Systematic Reviews. 2007;(2) doi: 10.1002/14651858.MR000012.pub2. [DOI] [PubMed] [Google Scholar]
- 7.Barton S. Which clinical studies provide the best evidence? The best RCT still trumps the best observational study. BMJ. 2000;321:255–256. doi: 10.1136/bmj.321.7256.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sackett DL, Rosenberg WM, Gray JA, Haynes RB, Richardson WS. Evidence based medicine: what it is and what it isn't. BMJ. 1996;312:71–72. doi: 10.1136/bmj.312.7023.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Treweek S, Pitkethly M, Cook J, Kjeldstrom M, Taskila T, Johansen M, et al. Strategies to improve recruitment to randomised controlled trials (Review) The Cochrane Library. 2010;4:1–10. doi: 10.1002/14651858.MR000013.pub5. [DOI] [PubMed] [Google Scholar]
- 10.Walson PD. Patient recruitment: US perspective. Pediatrics. 1999;104:619–622. [PubMed] [Google Scholar]
- 11.Easterbrook PJ, Matthews DR. Fate of research studies. J R Soc Med. 1992;85:71–76. doi: 10.1177/014107689208500206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Altman DG, Bland JM. Absence of evidence is not evidence of absence. Br Med J. 1995;311:485. doi: 10.1136/bmj.311.7003.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caldwell PHY, Hamilton S, Tan A, Craig JC. Strategies for increasing recruitment to randomised controlled trails: Systematic review. PLOS Medicine. 2010;7(11):1–16. doi: 10.1371/journal.pmed.1000368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zifferblatt SM. Recruitment in large-scale clinical trials. In: Weiss SM, editor. Proceeding of the National Heart and Lung Institute Working Conference on health behavior. Bethesda: NIH; pp. 187–195. [Google Scholar]
- 15.Charlson ME, Horwitz RI. Applying results of randomised trials to clinical practice: impact of losses before randomisation. British Medical Journal. 1984;289:1281–1284. doi: 10.1136/bmj.289.6454.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haidich AB, Ioannidis JPA. Patterns of patient enrolment in randomized controlled trials. Journal of Clinical Epidemiology. 2001;54:877–883. doi: 10.1016/s0895-4356(01)00353-5. [DOI] [PubMed] [Google Scholar]
- 17.Foy R, Parry J, Duggan A, Delaney B, Wilson S, Lewin-van den Broek NT, et al. How evidence-based are recruitment strategies for randomized controlled trials in primary care? Experience from seven studies Family Practice. 2003;20:83–92. doi: 10.1093/fampra/20.1.83. [DOI] [PubMed] [Google Scholar]
- 18.McDonald AM, Knight RC, Campbell MK, Entwistle VA, Grant AM, Cook JA, et al. What influences recruitment to randomised controlled trials? A review of trials funded by two UK funding agencies Trials. 2006;7:9. doi: 10.1186/1745-6215-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Agarwal A, Deepinder F, Cocuzza M, Agarwal A, Deepinder F, Cocuzza M, et al. Efficacy of varicocelectomy in improving semen parameters: new meta-analytical approach. Urol. 2007;70:532–538. doi: 10.1016/j.urology.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 20.Marmar JL, Agarwal A, Prabakaran S, Agarwal R, Short RA, Benoff S, et al. Reassessing the value of varicocelectomy as a treatment for male subfertility with a new meta-analysis. Fertil Steril. 2007;88:639–646. doi: 10.1016/j.fertnstert.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 21.Evers JL, Collins JA. Surgery or embolization for varicocele in subfertile men. Cochrane Database Syst Rev. 2004 doi: 10.1002/14651858.CD000479.pub2. [DOI] [PubMed] [Google Scholar]
- 22.Evers JL, Collins JA. Assessment of efficacy of varicocele repair for male subfertility: a systematic review. Lancet. 2003;361:1849–1852. doi: 10.1016/S0140-6736(03)13503-9. [DOI] [PubMed] [Google Scholar]
- 23.Watson JM, Torgerson DJ. Increasing recruitment to randomized trails: a review of randomised controlled trails. BMC Med Res Meth. 6(34):1–9. doi: 10.1186/1471-2288-6-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Evers JLH, Collins J, Clarke J. Surgery or embolization for varicoceles in subfertile men (review) The Cochrane Library. 2010;11:1–20. doi: 10.1002/14651858.CD000479.pub5. [DOI] [PubMed] [Google Scholar]
- 25.Will MA, Swain J, Fode M, Sonksen J, Christman GM, et al. The great debate: varicocele treatment and impact on fertility. Fertil Steril. 2011;95(3):841–852. doi: 10.1016/j.fertnstert.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krause W, Muller HH, Schafer H, Weidner W. Does treatment of varicocele improve male fertility? Results of the “Deutsche Varikozelenstudie,” a multicentre study of 14 collaborating centres. Andrologia. 2002;34:164–171. doi: 10.1046/j.1439-0272.2002.00494.x. [DOI] [PubMed] [Google Scholar]
- 27.Nilson S, Edvinsson A, Nilson B. Improvement of semen and pregnancy rate after ligation and division of the internal spermatic vein: fact or fiction? Br J Urol. 1979;51:591–596. doi: 10.1111/j.1464-410x.1979.tb03609.x. [DOI] [PubMed] [Google Scholar]
- 28.Breznik R, Vlausavlievic V, Borko E. Treatment of varicocele and male fertility. Arch Androl. 1993;30:157–160. doi: 10.3109/01485019308987750. [DOI] [PubMed] [Google Scholar]
- 29.Madgar I, Weissenberg R, Lunenfeld B, Karasik A, Goldwasser B. Controlled trail of high spermatic vein ligation for varicocele in infertile men. Fertil Steril. 1995;63:120–124. doi: 10.1016/s0015-0282(16)57306-3. [DOI] [PubMed] [Google Scholar]
- 30.Nieschlag E, Hertle L, Fischedick A, Abshagen K, Behre HM. Update on treatment of varicocele: counseling as effective as occlusion of the vena spermatica. Hum Reprod. 1998;113:2147–2150. doi: 10.1093/humrep/13.8.2147. [DOI] [PubMed] [Google Scholar]
- 31.Yamamoto M, Hibi H, Hirata Y, Miyake K, Ishigaki T. Effect of varicocelectomy on sperm parameters and pregnancy rate in patients with subclinical varicocele: a randomized prospective controlled study. J Urol. 1996;155:1636–1638. [PubMed] [Google Scholar]


