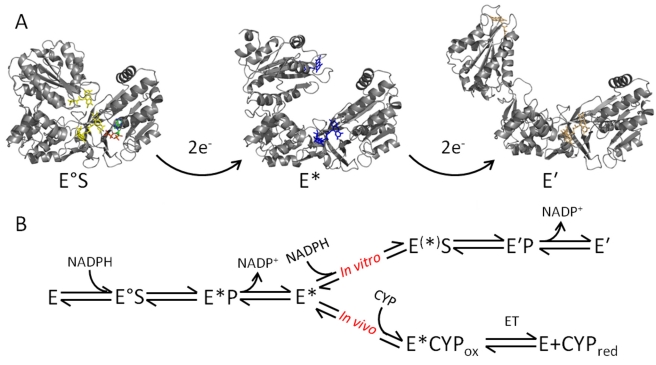
Figure 6. Integrated model of dynamics and chemistry in CPR in a proposed 2,4 reaction cycle as observed in vitro.
The structures shown are taken from homology models of available rat CPR X-ray crystal structures and are used to exemplify particular conformational changes. (A) CPR undergoes large scale domain motion subsequent to flavin reduction. There is a detectable conformational change associated with the 2- and 4-electron reduced state of CPR, giving the species E/E° (oxidized, yellow flavin), E* (2-electron reduced, blue flavin), and E′ (4-electron reduced, orange flavin). Binding of NADPH (green sticks) to oxidized (E) or 2-electron reduced (E*) CPR causes a relative closing to form E°S and E(*)S, respectively. (B) A model for directional transfer of electrons in vitro and in vivo. The 2-electron reduced form of CPR (E*) is capable of being further reduced (E′) in vitro. However, in vivo electrons are transferred from the open CPR (E*) to the heme of a CYP partner. A similar scheme for a 1,3 catalytic cycle is possible in vivo in which conversion of enzyme species E° to E* represents reduction of 1-electron reduced CPR to 3-electron reduced CPR (see text for details).