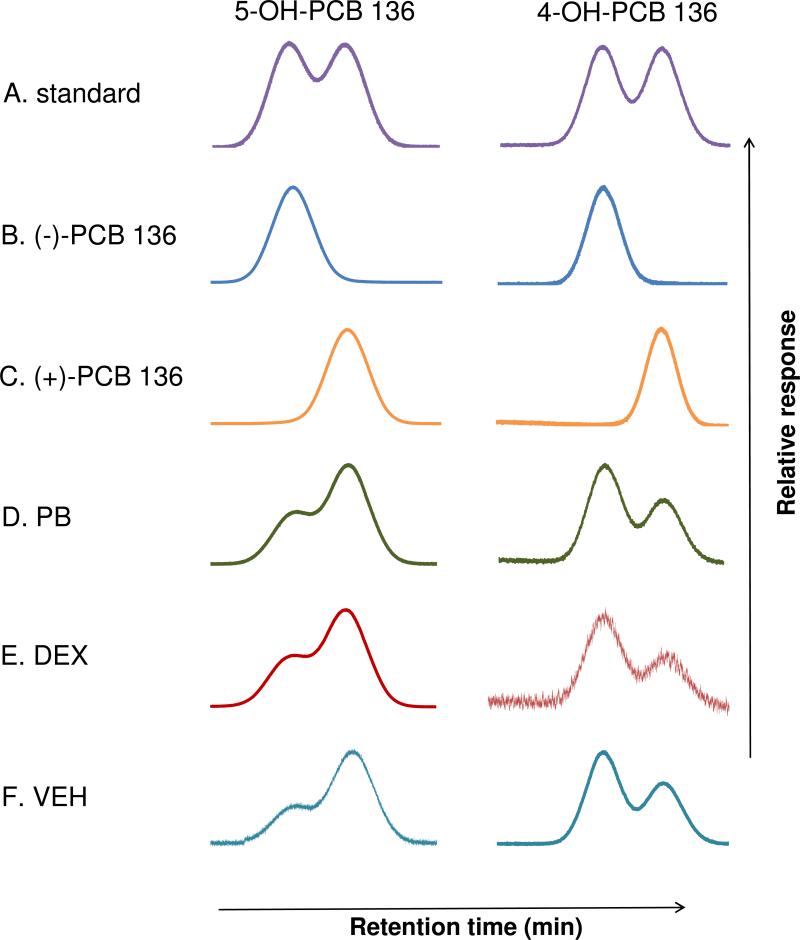
Figure 3.
The second eluting atropisomer of 5-OH PCB 136 (E(2)-5-OH-PCB 136) and the first eluting atropisomer of 4-OH PCB 136 (E(1)-4-OH-PCB) are formed enantioselectively by rat liver microsomes, with the first and second eluting atropisomer of both metabolites being formed from (-)-PCB 136 and (+)-PCB 136, respectively. Representative, normalized chromatograms of the methylated derivatives of (A) racemic 5-OH-PCB 136 and 4-OH-PCB 136 standards. (B) E(1)-5-OH-PCB 136 and E(1)-4-OH-PCB 136 formed from (-)-PCB 136. (C) E(2)-5-OH-PCB 136 and E(2)-4-OH-PCB 136 formed from (+)-PCB 136. Chiral signature of 5-OH-PCB 136 and 4-OH-PCB 136 obtained from incubations with hepatic microsomes from rats treated with (D) PB, (E) DEX or (F) VEH. All microsomal incubations were performed with 50 μM PCB 136 at 37 °C for 10 min. Atropisomers of 5-OH PCB 136 and 4-OH PCB 136 were separated on Chirasil-Dex and Cyclosil-B columns, respectively.