Abstract
Purpose
To characterize the level of depression in patients with ocular inflammatory disease and to determine predictors of depression in this population.
Design
Prospective cross-sectional survey and medical record review.
Methods
Participants were consecutive patients with noninfectious ocular inflammatory disease in a university-based tertiary referral center. Subjects were given the self-administered Beck Depression Inventory-II (BDI-II), National Eye Institute Visual Function Questionnaire-25 (NEI VFQ-25) and additional supplemental questions. Medical records were reviewed for clinical characteristics. Univariate analyses were conducted to compare clinical characteristics between patients with and without a positive screen for depression, and a multivariate regression model was performed to determine the most significant predictors of depression.
Results
Of the 104 participants, 26.9% screened positive for depression with the BDI-II. Of these subjects, only 39.3% had been previously diagnosed with depression. NEI VFQ-25 scores were significantly lower in depressed patients in all subscales except driving and color vision. Predictors of depression were inadequate emotional support, lower visual functioning (VFQ composite score), history of changing immunomodulatory treatment and current oral corticosteroid use.
Conclusions
Depression may be a significant but under recognized comorbid condition in patients with ocular inflammatory disease. Worse visual function was associated with depression. We recommend heightened awareness of potential depression in patients with ocular inflammatory disease.
Introduction
Uveitis affects people of all ages and accounts for up to 30,000 new cases of blindness annually and 10% of blindness in the United States.1, 2 It has been shown to negatively impact both physical and mental health.3, 4 Depressive symptoms and poorer self-reported visual functioning, as assessed with self-administered questionnaires, have been found in age-related macular degeneration, retinitis pigmentosa, and in newly diagnosed glaucoma patients.5–7 However, to date, there have been few studies looking at depression and vision-related quality of life in patients with ocular inflammatory diseases.4, 8
For patients with ocular inflammatory diseases, in addition to the burden of the disease and potential for vision loss, there are several factors that may contribute to the development of depression. The drugs used to treat ocular inflammatory diseases, including corticosteroids and other immunosuppressive agents, can cause mania, depression, and other behavioral changes.9 Furthermore, in diseases with chronic inflammation there is evidence that cytokines are able to cross the blood-brain-barrier and induce behavioral changes.10 Patients with chronic inflammatory diseases such as rheumatoid arthritis, atopy, Alzheimers disease and multiple sclerosis can have significant comorbid depression disproportionate to disease-specific disability.11 A combination of these factors may place patients with ocular inflammatory diseases at a greater risk for depression. The purpose of this study was to determine the level of depression in patients with ocular inflammatory disease, as measured by a self-administered depression screening questionnaire, and the risk factors associated with depression, including self-reported visual functioning and clinical characteristics.
Material and Methods
Participants
Consecutive patients between March 2010 and July 2010 seen in the Uveitis and Ocular Inflammatory Disease Clinic at the Francis I. Proctor Foundation, a tertiary care referral center at the University of California, San Francisco, were included in the study. Eligible individuals included patients at least 18 years of age with a diagnosis of non-infectious ocular inflammatory disease, who were English speaking and able to provide written informed consent. Patients seen at the Francis I. Proctor Foundation clinic undergo a standard evaluation, which includes screening for tuberculosis, syphilis, as well as targeted evaluation for other infectious and non-infectious causes of ocular inflammatory disease.
Design and Procedures
After informed consent was obtained, participants completed a self-administered depression screening questionnaire and vision related quality of life questionnaire, as well as a supplemental questionnaire. Participants were given the option to complete the questionnaires at the clinic or complete the questionnaires at home and return them by mail with a pre-paid, self-addressed envelope. Medical records were reviewed for each patient in order to gather general demographic information on age, gender and self-reported race. Additional chart review was conducted to gather information on type of ocular inflammatory disease (uveitis, scleritis, or mucus membrane pemphigoid (MMP)), location of disease, chronicity and recurrence of disease, associated systemic diseases, current best corrected visual acuity (BCVA), presence of active inflammation (defined as greater than 0.5+ anterior chamber cell, greater than 0.5+ vitreous haze, active vasculitis, retinitis, choroiditis, or active scleritis), and current and past treatments. The status (active or inactive), location and chronicity of ocular disease were described and recorded as outlined by the Standardization of Uveitis Nomenclature (SUN) criteria.12 Anatomic location of inflammation was noted as anterior, intermediate, posterior/panuveitis, scleritis, cicatrizing conjunctivitis or orbital inflammation. The type of uveitis was noted as acute (new onset and singular event), recurrent (multiple episodes of inflammation marked by medication-free inactive periods of greater than 3 months), or chronic (persistent inflammation and inability to remain free of inflammation for at least 3 months without medications). History of changing immunomodulatory therapy for any reason was recorded. Immunomodulatory medications are typically prescribed to patients with ocular inflammation using a stepladder approach, starting with antimetabolites and adding T-cell inhibitors and biologics for refractory cases. Alkylating agents are also used in particularly severe cases. The necessity to change therapies may result from poorly controlled inflammation, inability to taper concomitant corticosteroids, intolerance to an agent due to undesirable side effects or safety concerns, discontinuation secondary to cost, or reduced effectiveness of an agent over time, as can happen with TNFα inhibitors. Socioeconomic status, approximated by median household income, was determined by using geocodes (http://www.ffiec.gov/Geocode/default.aspx, accessed July 20, 2010).
The Beck Depression Inventory II (BDI-II), a validated 21 item self-administered questionnaire, was used to measure depression in this study.13–15 Each question has 4 choices, ranging in point value from 0 to 3. Total scores of 0 to 13 represent no depression, 14 to 19 mild depression, 20 to 28 moderate depression, and 29 to 63 severe depression.15–17 Using a cut-off value of >13 as a positive screen for depression yields 90% sensitivity and 99% specificity.13
Vision-related quality of life (VR-QOL) was determined using the National Eye Institute Visual Functioning Questionnaire (NEI VFQ-25), a self-administered 25 question survey. An overall composite score is generated with the lowest score of 0 to the highest score of 100, along with 12 subscales each with scores from 0 to 100 relating to general health, general vision, ocular pain, near activities, distance activities, social functioning, mental health, role difficulties, dependency, driving, color vision and peripheral vision.18, 19
An additional supplementary questionnaire was created to capture information regarding past history of depression, level of perceived emotional support, knowledge about uveitis and participation in uveitis support groups, and smoking history.
Statistical Considerations
Conservatively assuming that 10% of incoming patients would be classified as depressed,20 a sample size of 100 patients was chosen to give us 80% power to detect a 20-point difference in composite NEI-VFQ score using a two-sided t-test. This estimate assumes a 95% confidence level and a pooled standard deviation of 21.6 points.21 If as many as 25% were classified as depressed, as seen in another study of chronic eye disease,6 we would be able to detect a somewhat smaller difference of 14 points.
Statistical analyses were conducted using R statistical software. The Fisher exact test was used to determine differences in categorical variables between the depressed and not depressed groups, while continuous variables were analyzed using a two-sided t-test; all tests were performed at a 95% confidence level. Pearson correlations were included where appropriate. A backwards stepwise linear regression model using all variables was used to determine significant predictors of BDI-II score. A backwards stepwise logistic regression model using all variables was used to determine significant predictors of whether a subject screened positive or negative for depression according to BDI-II. Visual acuity using a Snellen eye chart was recorded individually for each eye and then converted into logarithm of the minimum angle of resolution (LogMAR) scale. Low vision of count fingers, hand motion, light perception and no light perception was recorded as logMAR 1.7, 1.8, 1.9 and 2.0, respectively.22 Institutional Review Board (IRB)/Ethics Committee approval was obtained for this study. The conduct of this study was HIPAA compliant.
Results
Of 151 eligible subjects for the study, 31 declined, 8 consented but did not mail in their questionnaires, and 8 consented but had incomplete questionnaires, resulting in 104 consecutive patients in the analysis. Twenty-eight (26.9%) subjects scored greater than 13 on the BDI-II (“depressed group”) and 76 (73.1%) subjects scored 13 or less (“not depressed group”). There were no significant demographic differences between the depressed and not depressed groups. Overall, 54.8% of participants were female and 49.0% were Caucasian (Table 1). Inflammation-specific clinical characteristics were compared between the depressed and non-depressed groups. Twenty-four (85.7%) subjects in the depressed group and 44 (57.9%) subjects in the not depressed group had chronic ocular inflammatory disease (P=0.01). Twenty-five (89.3%) subjects in the depressed group and 52 (68.4%) subjects in the not depressed group had bilateral disease (P=0.04). Fifteen (53.5%) subjects in the depressed group and 16 (21.1%) in the not depressed group were currently taking oral corticosteroids (P=0.003). Twelve (42.9%) in the depressed group and 13 (17.1%) in the not depressed group were currently taking an antimetabolite (P=0.01). Nine (32.1%) subjects in the depressed group and 6 (7.9%) subjects in the not depressed group had been treated with more than one immunomodulatory therapy (P=0.004).
TABLE 1.
Demographics, clinical characteristics and depression screeninga results of 104 patients with ocular inflammatory disease.
| Overall (N=104) | Depressed (N=28) | Not Depressed (N=76) | P-value | |
|---|---|---|---|---|
| Demographics | ||||
| Female | 57 (54.8%) | 16 (57.1%) | 41 (53.9%) | .83b |
| Race | .01b | |||
| Caucasian | 51 (49.0%) | 12 (42.9%) | 39 (51.3%) | |
| Hispanic | 11 (10.6%) | 8 (28.6%) | 3 (3.9%) | |
| Asian | 23 (22.1%) | 3 (10.7%) | 20 (26.3%) | |
| Black | 10 (9.6%) | 2 (7.1%) | 8 (10.5%) | |
| Indian Subcontinent | 6 (5.8%) | 1 (3.6%) | 5 (6.6%) | |
| Native American | 2 (1.9%) | 1 (3.6%) | 1 (1.3%) | |
| Other | 1 (1.0%) | 1 (3.6%) | 0 (0.0%) | |
| Current smoker | 9 (8.7%) | 2 (7.1%) | 7 (9.2%) | .95b |
| Median household income in dollars (IQRd) | 94293 (77255, 121348) | 80268 (73158, 105766) | 95919 (81114, 125831) | .06c |
| Median age in years (IQRd) | 41.0 (32.0, 51.3) | 41.5 (30.8, 51.3) | 40.5 (32.8, 50.5) | .79c |
| Mean logMAR vision in the better eye (range) | 0.07 (−0.14, 0.6) | 0.12 (0, 0.54) | 0.05 (−0.14, 0.6) | .02c |
| Any systemic inflammatory disease | 42 (40.4%) | 13 (46.4%) | 29 (38.2%) | .50b |
| Ankylosing spondylitis | 12 (11.5%) | 1 (3.6%) | 11 (14.5%) | |
| Behcet’s disease | 1 (1.0%) | 0 (0%) | 1 (1.3%) | |
| Inflammatory bowel disease | 2 (1.9%) | 1 (3.6%) | 1 (1.3%) | |
| Juvenile idiopathic arthritis | 1 (1.0%) | 1 (3.6%) | 0 (0%) | |
| Mucous membrane pemphigoid (MMP) | 3 (2.9%) | 2 (7.1%) | 1 (1.3%) | |
| Multiple sclerosis | 1 (1.0%) | 1 (3.6%) | 0 (0%) | |
| Parry Romberg syndrome | 1 (1.0%) | 0 (0%) | 1 (1.3%) | |
| Reactive arthritis | 2 (1.9%) | 1 (3.6%) | 1 (1.3%) | |
| Rheumatoid arthritis | 2 (1.9%) | 1 (3.6%) | 1 (1.3%) | |
| Rosacea | 1 (1.0%) | 0 (0%) | 1 (1.3%) | |
| Sarcoidosis | 7 (6.7%) | 1 (3.6%) | 6 (7.9%) | |
| Systemic lupus erythematosus | 2 (1.9%) | 0 (0%) | 2 (2.6%) | |
| Tubulointerstitial nephritis and uveitis | 1 (1.0%) | 0 (0%) | 1 (1.3%) | |
| Vogt-Koyanagi-Harada syndrome | 6 (5.8%) | 4 (14.3%) | 2 (2.6%) | |
|
| ||||
| Clinical Course | ||||
| Median duration of disease in years (IQRd) | 5.8 (2.0, 12.0) | 3.9 (1.6, 9.3) | 6.3 (3.0, 12.3) | .18c |
| Chronic | 68 (65.4%) | 24 (85.7%) | 44 (57.9%) | .01b |
| Bilateral | 77 (74.0%) | 25 (89.3%) | 52 (68.4%) | .04b |
| Location of Inflammation | .01b | |||
| Anterior | 49 (47.1%) | 8 (28.6%) | 41 (53.9%) | |
| Intermediate | 8 (7.7%) | 0 (0%) | 8 (10.5%) | |
| Anterior + intermediate | 11 (10.6%) | 6 (21.4%) | 5 (6.6%) | |
| Posterior/Pan | 27 (25.9%) | 11 (39.2%) | 16 (21.1%) | |
| Scleritis | 4 (3.8%) | 1 (3.5%) | 3 (3.9%) | |
| Conjunctiva (MMP) | 3 (2.9%) | 2 (7.1%) | 1 (1.3%) | |
| Cornea (peripheral ulcerative keratitis) | 1 (1.0%) | 0 (0%) | 1 (1.3%) | |
| Orbit | 1 (1.0%) | 0 (0%) | 1 (1.3%) | |
| Current active inflammation | 26 (25.0%) | 7 (25.0%) | 19 (25.0%) | .99b |
|
| ||||
| Current Treatment | ||||
| Oral corticosteroid | 31 (29.8%) | 15 (53.5%) | 16 (21.1%) | .003b |
| Topical corticosteroid | 51 (49.0%) | 13 (46.4%) | 38 (50.0%) | .92b |
| Antimetabolite | 25 (24.0%) | 12 (42.9%) | 13 (17.1%) | .01b |
| Methotrexate | 13 (12.5%) | 6 (21.4%) | 7 (9.2%) | |
| Mycophenolate mofetil | 11 (10.6%) | 6 (21.4%) | 5 (6.6%) | |
| Azathioprine | 1 (1.0%) | 0 (0%) | 1 (1.3%) | |
| Cyclosporine | 1 (1.0%) | 1 (3.6%) | 0 (0%) | .27b |
| Cyclophosphamide | 2 (1.9%) | 2 (7.1%) | 0 (0%) | .07b |
| Biologic | 10 (9.6%) | 3 (10.7%) | 7 (9.2%) | .99b |
| Infliximab | 6 (5.8%) | 1 (3.6%) | 5 (6.6%) | |
| Adalimumab | 4 (3.8%) | 2 (7.1%) | 2 (2.6%) | |
| History of changing immunomodulatory therapy | 15 (14.4%) | 9 (32.1%) | 6 (7.9%) | .004b |
Beck Depression Inventory II: patients in the depressed group scored >13 and patients in the not depressed group scored ≤13
Analyzed using a Fisher Exact Test at a confidence level of 95%
Analyzed using a two-sided t-test at a confidence level of 95%
Interquartile range
In the depressed group, 13 patients had a BDI-II score of 14–19 (mild depression), 11 patients had a score of 20–28 (moderate depression), and 4 patients had a score >28 (severe depression). The mean BDI-II score in the depressed group was 21.8 (range 14–40) and the mean BDI-II score in the not depressed group was 4.5 (range 0–12) (Figure 1). The mean logMAR vision in the better seeing eye was 0.12 (Snellen equivalent 20/25-) in the depressed group and 0.05 (Snellen equivalent 20/25+2) in the not depressed group (P=0.02) (Table 1). The mean composite NEI VFQ-25 scores for the depressed and not depressed groups were 63.6 and 85.6, respectively (P<0.001) (Table 2). All subscale scores were significantly lower in the depressed group except for driving and color vision. There was a moderate inverse correlation between BDI-II score and VFQ composite score (Pearson correlation −0.53, P<0.001) and between logMAR BCVA in the better seeing eye and VFQ composite score (Pearson correlation −0.58, P<0.001) (Figures 2 and 3). Among patients with anterior, anterior+intermediate, or posterior/pan uveitis, logMAR BCVA in the better seeing eye was slightly lower in the depressed group but not significantly different (Table 3). VFQ composite scores were significantly lower for depressed patients with anterior+intermediate (P=0.006) and posterior/pan uveitis (P=0.03), but not for depressed patients with strictly anterior uveitis (P=0.30).
FIGURE 1.
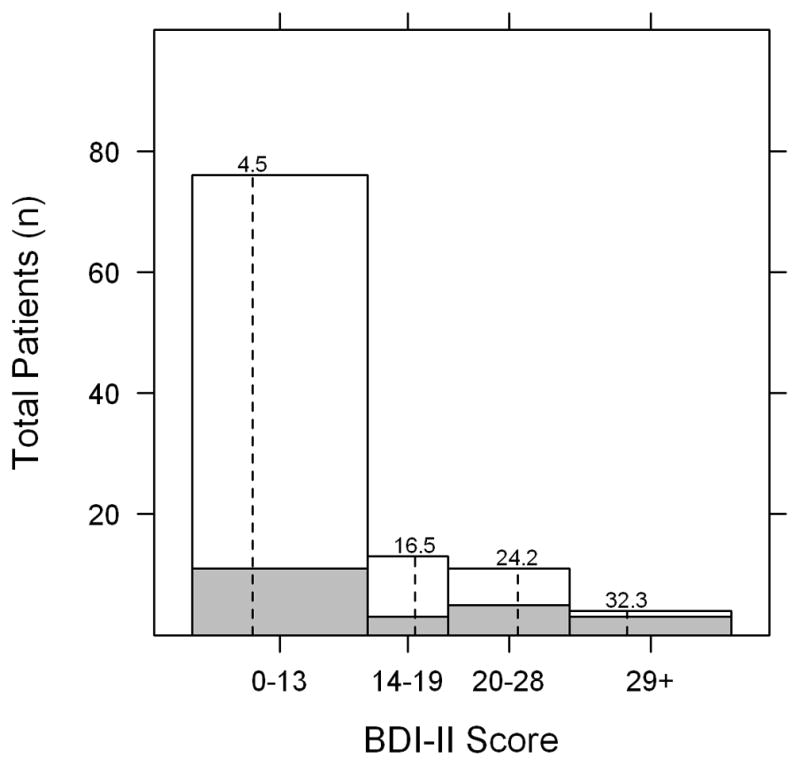
Distribution of Beck Depression Inventory II (BDI-II) scores among 104 patients with ocular inflammatory disease. Scores of 0–13 represent no depression, 14–19 mild depression, 20–28 moderate depression and 29 and over severe depression. Dashed lines represent the mean BDI-II scores within each depression category. Shaded areas represent patients who had self-reported diagnoses of clinical depression.
Table 2.
Vision-related quality of life assessed by the NEI VFQ-25a and depression screeningb results among patients with ocular inflammatory disease
| Overall | Depressed | Not Depressed | P-valuec | |
|---|---|---|---|---|
| Mean composite score (range) | 79.7 (16, 100) | 63.6 (16, 98.1) | 85.6 (31.1, 100) | <.001 |
| Mean subscale scores (range) | ||||
| General health | 60.3 (0, 100) | 46.4 (0, 75) | 65.5 (0, 100) | <.001 |
| General vision | 72.8 (20, 100) | 65.2 (20, 100) | 75.5 (20, 100) | .03 |
| Ocular pain | 73.9 (0,100) | 60.3 (12.5, 100) | 78.9 (0, 100) | .002 |
| Near activities | 79.2 (16.7, | 61.6 (16.7, 100) | 85.6 (16.7, 100) | <.001 |
| Distance activities | 78.8 (8.3, 100) | 59.8 (8.3, 100) | 85.7 (33.3, 100) | <.001 |
| Social functioning | 89.9 (25, 100) | 76.3 (25, 100) | 94.9 (37.5, 100) | <.001 |
| Mental health | 70.8 (0, 100) | 44.0 (0, 93.8) | 80.7 (12.5, 100) | <.001 |
| Role difficulties | 74.2 (0, 100) | 50.9 (0, 100) | 82.7 (0, 100) | <.001 |
| Dependency | 84.9 (8.3, 100) | 66.4 (8,3, 100) | 91.8 (25, 100) | <.001 |
| Driving | 77.4 (0, 100) | 62.0 (0, 100) | 82.4 (0, 100) | .007 |
| Color vision | 94.9 (25, 100) | 91.7 (25, 100) | 96.1 (25, 100) | .24 |
| Peripheral vision | 81.3 (0, 100) | 66.1 (0, 100) | 86.8 (25, 100) | .002 |
National Eye Institute Visual Functioning Questionnaire 25
Beck Depression Inventory II: patients in the depressed group scored >13 and patients in the not depressed group scored ≤13
Analyzed using a two-sided t-test at a confidence level of 95%
FIGURE 2.
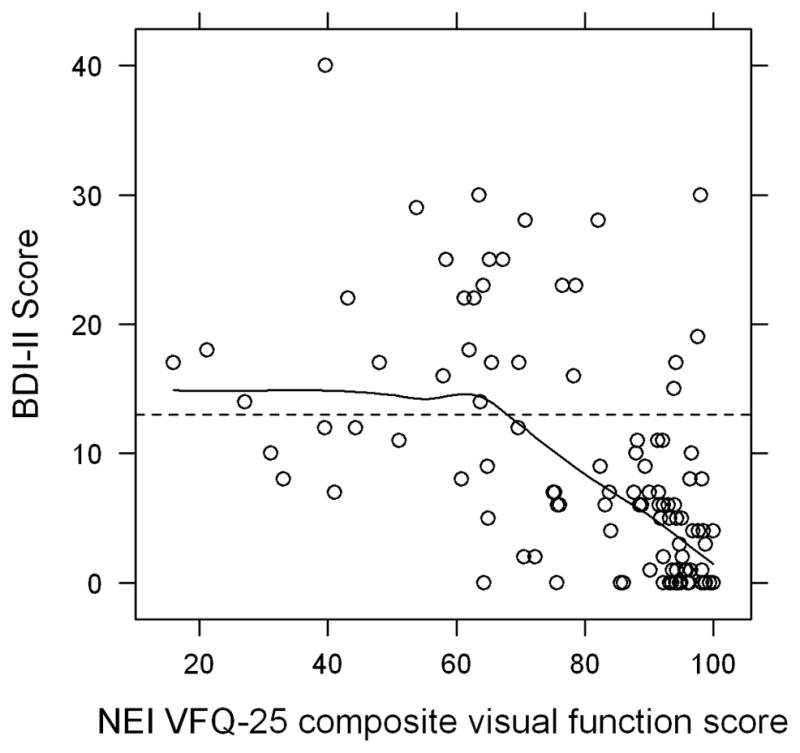
Correlation between Beck Depression Inventory II (BDI-II) score and National Eye Institute Visual Functioning Questionnaire (NEI VFQ-25) score in patients with ocular inflammatory disease. Dashed line represents the cutoff BDI-II score of 13 between the negative and positive screen. A Pearson correlation of −0.53 represents a moderate to strong inverse correlation (P < 0.001).
FIGURE 3.
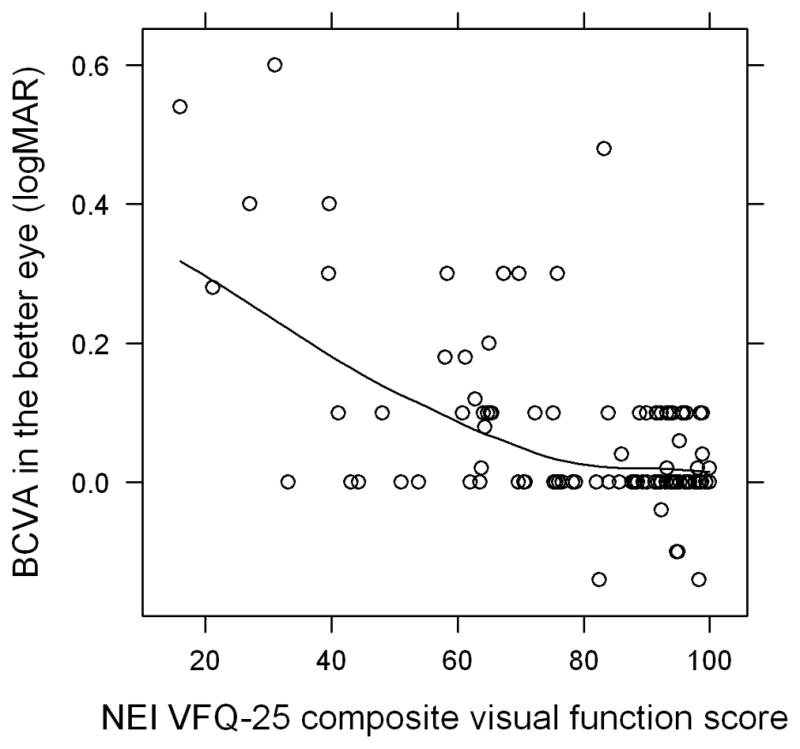
Correlation between best corrected visual acuity (BCVA) in logMAR in the better seeing eye and National Eye Institute Visual Functioning Questionnaire (NEI VFQ-25) composite score in patients with ocular inflammatory disease. A Pearson correlation of −0.58 (P < 0.001) represents a moderate to strong inverse correlation.
Table 3.
Vision-related quality of life assessed by the NEI VFQ-25a composite score, vision in the better seeing eye, and depression screeningb results among uveitis patients with various anatomical locations of inflammation
| Overall | Depressed | Not Depressed | P-valuec | |
|---|---|---|---|---|
| Anterior | N=49 | N=8 | N=41 | |
| Mean VFQ-25 composite score (range) | 85.2 (21.2, 100) | 76.1 (21.2, 98.1) | 86.9 (33.1, 100) | .30 |
| Mean logMAR vision in the better eye (range) | 0.04 (−0.14, 0.48) | 0.07 (0, 0.28) | 0.03 (−0.14, 0.60) | .37 |
|
| ||||
| Intermediate | N=8 | N=0 | N=8 | |
| Mean VFQ-25 composite score (range) | 85.6 (60.8, 100) | NA | 85.6 (60.8, 100) | NA |
| Mean logMAR vision in the better eye (range) | 0.09 (0, 0.30) | NA | 0.09 (0, 0.30) | NA |
|
| ||||
| Anterior + Intermediate | N=11 | N=6 | N=5 | |
| Mean VFQ-25 composite score (range) | 68.3 (27.1, 94.2) | 54.4 (27.1, 69.8) | 85.0 (64.8, 94.2) | .006 |
| Mean logMAR vision in the better eye (range) | 0.15 (0, 0.40) | 0.20 (0, 0.40) | 0.08 (0.02, 0.10) | .12 |
|
| ||||
| Posterior/Panuveitis | N=27 | N=11 | N=16 | |
| Mean VFQ-25 composite score (range) | 73.7 (15.9, 99.4) | 62.7 (15.9, 82.1) | 81.3 (31.1, 99.43) | .03 |
| Mean logMAR vision in the better eye (range) | 0.09 (−0.04, 0.60) | 0.11 (0, 0.54) | 0.07 (−0.04, 0.60) | .54 |
National Eye Institute Visual Functioning Questionnaire 25
Beck Depression Inventory II: patients in the depressed group scored >13 and patients in the not depressed group scored ≤13
Analyzed using a two-sided t-test at a confidence level of 95%
NA=not applicable
Of the nine supplemental questions administered, three had significantly different responses between the depressed and not depressed groups: 39.3% of patients in the depressed group versus 14.5% in the not depressed group had previously been diagnosed with depression (P=0.01); 42.8% of the depressed group versus 18.4% of the not depressed group had been prescribed an antidepressant medication at some point (P=0.02); 50.0% of the depressed group versus 92.1% of the not depressed group reported receiving adequate emotional support from the people close to them (P<0.001). Of all 104 subjects surveyed, 19.2% reported knowing someone else who had ocular inflammatory disease; 47.1% thought being part of an ocular inflammatory disease support group would be helpful but only 15.4% actually belonged to one, and 80.8% used the internet to learn more about ocular inflammatory disease and 68.3% thought doing so was helpful. None of these responses were significantly different between the depressed and non-depressed groups.
In multivariate analysis using backward stepwise linear regression, the following associations between predictors and BDI-II numeric score were statistically significant: for every 10 point increase in VFQ composite score, BDI-II score decreased by 1.6 points (95% confidence interval (CI) −2.25 to −0.87); for every 10 point increase in VFQ general health score, BDI-II score decreased by 1.1 points (95% CI −1.67 to −0.61); subjects with a history of switching immunomodulatory therapy for any reason had increased BDI-II scores by 8.1 points (95% CI 4.44 to 11.70); and subjects with bilateral disease had increased BDI-II scores by 3.2 points (95% CI 0.31 to 6.11) (Table 4). In a logistic regression model, the following variables were retained as significant predictors of whether or not a subject scored greater than 13 (positive depression screen) on the BDI-II: perceived adequate emotional support (odds ratio 0.09, 95% CI 0.02–0.35), 10-point change in VFQ composite score (odds ratio 0.67, 95% CI 0.49–0.89), history of switching immunomodulatory therapy (odds ratio 6.97, 95% CI 1.49–36.13) and current use of oral corticosteroid (odds ratio 3.78, 95% CI 1.08–14.47).
TABLE 4a.
Multivariate linear regression model predicting BDI-IIa score in patients with ocular inflammatory disease
| Variable | Coefficient | 95% CIc | P-value |
|---|---|---|---|
| Intercept | 25.27 | (19.55, 30.99) | <.001 |
| NEI VFQ-25b composite score | −1.56 | (−2.25, −0.87) | <.001 |
| NEI VFQ-25b general health score | −1.14 | (−1.67, −0.61) | <.001 |
| History of changing immunomodulatory therapy | 8.07 | (4.44, 11.70) | <.001 |
| Bilateral disease | 3.21 | (0.31, 6.11) | .03 |
Beck Depression Inventory II: patients in the depressed group scored >13 and patients in the not depressed group scored ≤13
National Eye Institute Visual Functioning Questionnaire 25: a unit change in NEI VFQ-25 reflects a 10-point change in score in this model
Confidence interval
Discussion
Twenty-eight of 104 subjects with ocular inflammatory disease (26.9%) screened positive for depression by the BDI-II in our tertiary practice, which is much higher than 10% in the general population.20 This is in the same range as patients with chronic diseases such as rheumatoid arthritis,23 diabetes,24 cancer25 and other chronic eye diseases such as retinitis pigmentosa6 and advanced macular degeneration.5 Less is known about depression in patients with ocular inflammatory disease. One recent study reported markedly decreased health-related quality of life, as measured by the Medical Outcomes Study 36-Item Short Form Questionnaire (SF-36), in patients with uveitis on chronic systemic immunosuppressive treatment.3 Another study on HLA-B27-associated uveitis patients found that these patients had more depressive symptoms and negative coping strategies than controls, perhaps implicating stress and life events as a trigger for relapses.26 However, to our knowledge, this is the first prospective cross-sectional study evaluating prevalence of depression in a practice-based population of patients with ocular inflammatory disease and assessing risk factors for a positive depression screen.
One of our most striking findings is that only 39.3% of subjects with a positive depression screen had been diagnosed with depression, by self-report. Moreover, 11 subjects had BDI-II scores indicating moderate depression and 4 subjects had scores indicating severe depression. Although a positive screen does not replace a formal psychiatric evaluation, these data are highly suggestive that depression may be vastly under diagnosed and by extension undertreated in our study population. Under diagnosis and under treatment of depression has also been recognized in patients with rheumatoid arthritis and noted to be a barrier to effective treatment of the underlying disease.27
Another significant finding is that although self-reported visual function and clinically measured visual acuity are both associated with higher BDI-II scores, composite VFQ score is identified as a better predictor by multivariate regression. This supports previous findings in the literature. In a cross-sectional study of data from the randomized Collaborative Initial Glaucoma Treatment Study (CIGTS), poorer self-reported visual function was correlated with more symptoms of depression and poor mood, whereas objective measures of visual function such as visual acuity and visual field were not.7 Likewise, in community-dwelling elderly adults with advanced macular degeneration, the relationship between disability and depression was strong whereas that between visual acuity and depression was weak.5 These studies support our clinical impression that Snellen acuity does not fully characterize visual disability. In fact, we found the NEI VFQ-25 scores to be substantially worse in almost all subscales in the depressed group compared to the not depressed group.
The NEI VFQ-25 scores (composite and subscales) in our study population are in the same range as those found in uveitis patients studied at the National Eye Institute and in a cohort of patients with birdshot chorioretinopathy.4,21 In the NEI study, all VFQ scores were significantly lower in uveitis patients compared to the reference group of normal subjects. In addition, we found a difference of 22 points in the NEI VFQ-25 composite score between depressed and non-depressed patients. Slightly smaller differences have been found in other studies of patients with macular degeneration (11.2 points),5 retinitis pigmentosa (15.5 points),6 and a population-based Latino eye study (11.8 points).28
Fifty percent of subjects in the depressed group self-reported adequate emotional support from people close to them, compared to an overwhelming 92.1% of subjects in the not depressed group. In fact, subjects receiving adequate emotional support were 11.1 times less likely than subjects not receiving adequate emotional support to screen positive for depression, making this the strongest and most significant predictor against a positive depression screen. Adequate emotional support may be protective for depression, or feelings of isolation may simply be prominent symptoms of depression.
Other factors that are traditionally thought to contribute to depression include burden of chronic disease and side effects of systemic immunosuppressive medications.9, 29 Even though patients often report anecdotal decrease in mood with longer duration of disease and current activity of disease, we found no correlation between active inflammation and a positive depression screen (P = 0.99) or between duration of disease and depression (P = 0.18). Although active inflammation at the time of the depression screen was not predictive of a positive screen, severity of disease is difficult to capture at any given time point since inflammation is dynamic and is dependent on current treatments that may mask uveitis, such as corticosteroids. However, a history of switching immunomodulatory therapy was predictive of depression, perhaps indicating patients with more refractory disease are at greater risk. We did find on univariate analysis that current oral corticosteroid and antimetabolite use were associated with a positive depression screen, although these two variables were not significant on multivariate analysis.
Most recently, there is evidence that a chronic inflammatory state and dysregulation of cytokines may play a role in the pathophysiology of depression. Cytokines are reported to cross the blood brain barrier and cause sickness behavior.10, 30 Depressed patients have higher levels of circulating cytokines IL-1β, IL-6, INF-γ, and TNF-α compared to controls.31 Thus, the proinflammatory cytokines IL-1 and TNF-α are potential targets for therapy.32 Indeed, 12 weeks of etanercept led to improvements in depressive symptoms and BDI-II scores in 618 patients with psoriasis.33 However, the small number of subjects taking TNF-α inhibitors in our study does not allow us to evaluate this association.
There are several limitations of our study. We used a self-administered questionnaire and did not have formal psychiatric evaluation to confirm the diagnosis of depression, but the Beck Depression Inventory II has been validated as a screening tool for depression. BDI-II focuses only on mood dysfunction and does not assess other important aspects of depression such as cognitive function, which may be impaired among patients with depression but was not assessed as part of this study.34–36 Because of the tertiary nature of our practice, there is an inherent selection bias for patients with chronic, recalcitrant ocular inflammatory disease necessitating systemic treatment, so depressive symptoms and visual dysfunction may be greater in our patient population than other clinics. In addition, with multiple comparisons, there is an increased chance of finding an association, so our results should be viewed as hypothesis-generating.
In summary, depression appears to be a major comorbidity in patients with ocular inflammatory disease, and the majority of patients screening positive have not been previously diagnosed. Depression screening could be considered in this patient population. Alternatively, readily available clinical information such as having chronic inflammation or switching immunomodulatory therapy may be helpful in identifying patients at risk for depression. There also may be utility in identifying patients with low self-reported visual function and inadequate emotional support. We recommend heightened awareness of potential depression in patients with ocular inflammatory disease.
Table 4b.
Multivariate logistic regression model predicting BDI-IIa score in patients with ocular inflammatory disease
| Variable | Odds | 95% CIc | P-value |
|---|---|---|---|
| Adequate emotional support | 0.09 | (0.02, 0.35) | <.001 |
| NEI VFQ-25b composite score | 0.67 | (0.49, 0.89) | .007 |
| History of changing immunomodulatory therapy | 6.97 | (1.49, 36.13) | .02 |
| Oral corticosteroid (current) | 3.78 | (1.08, 14.47) | .04 |
Beck Depression Inventory II: patients in the depressed group scored >13 and patients in the not depressed group scored ≤13
National Eye Institute Visual Functioning Questionnaire 25: a unit change in NEI VFQ-25 reflects a 10-point change in score in this model
Confidence interval
Acknowledgments
Financial support: This research was supported by a National Eye Institute K23EY017897 grant and a Research to Prevent Blindness Career Development Award to Dr. Acharya. This work was also supported by departmental grants NEI EY06190, That Man May See Foundation, and an unrestricted grant from the Research to Prevent Blindness Foundation. The sponsors had no role in the design or conduct of the study, data analysis or manuscript preparation. Nisha R. Acharya, MD, MS had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. The sponsor or funding organization had no role in the design or conduct of this research.
Biographies

Ying Qian, MD, received her medical degree from University of Pennsylvania School of Medicine in Philadelphia and completed her ophthalmology residency at Cole Eye Institute at Cleveland Clinic in Ohio. She completed a uveitis fellowship at the Francis I. Proctor Foundation at University of California San Francisco and will pursue a cornea fellowship at Proctor/UCSF. Her research interests include epidemiological studies in uveitis, corneal infections and tele-ophthalmology.

Nisha Acharya, MD, MS, is Director of the Uveitis Service at the F.I. Proctor Foundation and Associate Professor in the Department of Ophthalmology at the University of California, San Francisco. Her research interests include designing and implementing clinical trials and epidemiologic studies in the field of ocular infection and inflammation.
Footnotes
Financial Disclosures: No authors have any financial/conflicting interests to disclose.
Contributions of Authors: Design of the study (YQ, NRA); Conduct of the study (YQ, TG, NRA); Collection, management, analysis, and interpretation of the data (YQ, TG, EE, NRA); Preparation, review or approval of the manuscript (YQ, TG, EE, NRA).
Conformity Statement: The University of California San Francisco Institutional Review Board (IRB)/Ethics Committee approval was prospectively obtained for this study. Informed consent was obtained from all subjects. The conduct of this study was HIPAA compliant.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nussenblatt RB. The natural history of uveitis. Int Ophthalmol. 1990;14(5–6):303–308. doi: 10.1007/BF00163549. [DOI] [PubMed] [Google Scholar]
- 2.Suttorp-Schulten MS, Rothova A. The possible impact of uveitis in blindness: a literature survey. Br J Ophthalmol. 1996;80(9):844–848. doi: 10.1136/bjo.80.9.844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miserocchi E, Modorati G, Mosconi P, Colucci A, Bandello F. Quality of life in patients with uveitis on chronic systemic immunosuppressive treatment. Ocul Immunol Inflamm. 2010;18(4):297–304. doi: 10.3109/09273941003637510. [DOI] [PubMed] [Google Scholar]
- 4.Schiffman RM, Jacobsen G, Whitcup SM. Visual functioning and general health status in patients with uveitis. Arch Ophthalmol. 2001;119(6):841–849. doi: 10.1001/archopht.119.6.841. [DOI] [PubMed] [Google Scholar]
- 5.Brody BL, Gamst AC, Williams RA, et al. Depression, visual acuity, comorbidity, and disability associated with age-related macular degeneration. Ophthalmology. 2001;108(10):1893–1900. doi: 10.1016/s0161-6420(01)00754-0. discussion 1900–1901. [DOI] [PubMed] [Google Scholar]
- 6.Hahm BJ, Shin YW, Shim EJ, et al. Depression and the vision-related quality of life in patients with retinitis pigmentosa. Br J Ophthalmol. 2008;92(5):650–654. doi: 10.1136/bjo.2007.127092. [DOI] [PubMed] [Google Scholar]
- 7.Jampel HD, Frick KD, Janz NK, et al. Depression and mood indicators in newly diagnosed glaucoma patients. Am J Ophthalmol. 2007;144(2):238–244. doi: 10.1016/j.ajo.2007.04.048. [DOI] [PubMed] [Google Scholar]
- 8.Franke GH, Schutte E, Heiligenhaus A. Rehabilitation-psychological aspects of uveitis. Psychother Psychosom Med Psychol. 2005;55(2):65–71. doi: 10.1055/s-2004-828504. [DOI] [PubMed] [Google Scholar]
- 9.Warrington TP, Bostwick JM. Psychiatric adverse effects of corticosteroids. Mayo Clin Proc. 2006;81(10):1361–1367. doi: 10.4065/81.10.1361. [DOI] [PubMed] [Google Scholar]
- 10.Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9(1):46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pollak Y, Yirmiya R. Cytokine-induced changes in mood and behaviour: implications for ‘depression due to a general medical condition,’ immunotherapy and antidepressive treatment. The International Journal of Neuropsychopharmacology. 2002;5(4):389–399. doi: 10.1017/S1461145702003152. [DOI] [PubMed] [Google Scholar]
- 12.Jabs DA, Nussenblatt RB, Rosenbaum JT. Standardization of Uveitis Nomenclature for Reporting Clinical Data. Results of the First International Workshop. Am J Ophthalmol. 2005;140(3):509–516. doi: 10.1016/j.ajo.2005.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lasa L, Ayuso-Mateos JL, Vazquez-Barquero JL, Diez-Manrique FJ, Dowrick CF. The use of the Beck Depression Inventory to screen for depression in the general population: a preliminary analysis. J Affect Disord. 2000;57(1–3):261–265. doi: 10.1016/s0165-0327(99)00088-9. [DOI] [PubMed] [Google Scholar]
- 14.Arnau RC, Meagher MW, Norris MP, Bramson R. Psychometric evaluation of the Beck Depression Inventory-II with primary care medical patients. Health Psychol. 2001;20(2):112–119. doi: 10.1037//0278-6133.20.2.112. [DOI] [PubMed] [Google Scholar]
- 15.Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory. San Antonio: The Psychological Corporation; 1996. [Google Scholar]
- 16.Beck AT, Steer RA, Ball R, Ranieri W. Comparison of Beck Depression Inventories -IA and -II in psychiatric outpatients. J Pers Assess. 1996;67(3):588–597. doi: 10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- 17.Dozois DJA, Dobson KS, Ahnberg JL. A psychometric evaluation of the Beck Depression Inventory - II. Psychol Assess. 1998;10(2):83–89. [Google Scholar]
- 18.Mangione CM, Lee PP, Gutierrez PR, Spritzer K, Berry S, Hays RD. Development of the 25-item National Eye Institute Visual Function Questionnaire. Arch Ophthalmol. 2001;119(7):1050–1058. doi: 10.1001/archopht.119.7.1050. [DOI] [PubMed] [Google Scholar]
- 19.Mangione CM, Lee PP, Pitts J, Gutierrez PR, Berry S, Hays RD. Psychometric Properties of the National Eye Institute Visual Function Questionnaire (NEI-VFQ) Arch Ophthalmol. 1998;116(11):1496–1504. doi: 10.1001/archopht.116.11.1496. [DOI] [PubMed] [Google Scholar]
- 20.Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):617–627. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levinson RD, Monnet D, Yu F, Holland GN, Gutierrez P, Brezin AP. Longitudinal cohort study of patients with birdshot chorioretinopathy. V. Quality of life at baseline. Am J Ophthalmol. 2009;147(2):346–350. e342. doi: 10.1016/j.ajo.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 22.Wilhelmus KR, Gee L, Hauck WW, et al. Herpetic Eye Disease Study. A controlled trial of topical corticosteroids for herpes simplex stromal keratitis. Ophthalmology. 1994;101(12):1883–1895. doi: 10.1016/s0161-6420(94)31087-6. discussion 1895–1886. [DOI] [PubMed] [Google Scholar]
- 23.Margaretten M, Yelin E, Imboden J, et al. Predictors of depression in a multiethnic cohort of patients with rheumatoid arthritis. Arthritis Rheum. 2009;61(11):1586–1591. doi: 10.1002/art.24822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anderson RJ, Freedland KE, Clouse RE, Lustman PJ. The prevalence of comorbid depression in adults with diabetes: a meta-analysis. Diabetes Care. 2001;24(6):1069–1078. doi: 10.2337/diacare.24.6.1069. [DOI] [PubMed] [Google Scholar]
- 25.Massie MJ. Prevalence of depression in patients with cancer. J Natl Cancer Inst Monogr. 2004;2004(32):57–71. doi: 10.1093/jncimonographs/lgh014. [DOI] [PubMed] [Google Scholar]
- 26.Maca SM, Schiesser AW, Sobala A, et al. Distress, depression and coping in HLA-B27-associated anterior uveitis with focus on gender differences. Br J Ophthalmol. 2011;95(5):699–704. doi: 10.1136/bjo.2009.174839. [DOI] [PubMed] [Google Scholar]
- 27.Hider SL, Tanveer W, Brownfield A, Mattey DL, Packham JC. Depression in RA patients treated with anti-TNF is common and under-recognized in the rheumatology clinic. Rheumatology (Oxford) 2009;48(9):1152–1154. doi: 10.1093/rheumatology/kep170. [DOI] [PubMed] [Google Scholar]
- 28.Paz SH, Globe DR, Wu J, Azen SP, Varma R. Relationship between self-reported depression and self-reported visual function in Latinos. Arch Ophthalmol. 2003;121(7):1021–1027. doi: 10.1001/archopht.121.7.1021. [DOI] [PubMed] [Google Scholar]
- 29.Draper HM. Depressive disorder associated with mycophenolate mofetil. Pharmacotherapy. 2008;28(1):136–139. doi: 10.1592/phco.28.1.136. [DOI] [PubMed] [Google Scholar]
- 30.Pucak ML, Kaplin AI. Unkind cytokines: current evidence for the potential role of cytokines in immune-mediated depression. Int Rev Psychiatry. 2005;17(6):477–483. doi: 10.1080/02646830500381757. [DOI] [PubMed] [Google Scholar]
- 31.Zunszain PA, Anacker C, Cattaneo A, Carvalho LA, Pariante CM. Glucocorticoids, cytokines and brain abnormalities in depression. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35(3):722–729. doi: 10.1016/j.pnpbp.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Irwin MR, Miller AH. Depressive disorders and immunity: 20 years of progress and discovery. Brain Behav Immun. 2007;21(4):374–383. doi: 10.1016/j.bbi.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 33.Tyring S, Gottlieb A, Papp K, et al. Etanercept and clinical outcomes, fatigue, and depression in psoriasis: double-blind placebo-controlled randomised phase III trial. Lancet. 2006;367(9504):29–35. doi: 10.1016/S0140-6736(05)67763-X. [DOI] [PubMed] [Google Scholar]
- 34.Austin MP, Ross M, Murray C, O’Carroll RE, Ebmeier KP, Goodwin GM. Cognitive function in major depression. J Affect Disord. 1992;25(1):21–29. doi: 10.1016/0165-0327(92)90089-o. [DOI] [PubMed] [Google Scholar]
- 35.McClintock SM, Cullum M, Husain MM, et al. Evaluation of the Effects od Severe Depression on Global Cognitive Function and Memory. CNS spectrums. 2010;15(5):304–313. doi: 10.1017/s109285290002753x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McClintock SM, Husain MM, Greer TL, Cullum CM. Association between depression severity and neurocognitive function in major depressive disorder: a review and synthesis. Neuropsychology. 2010;24(1):9–34. doi: 10.1037/a0017336. [DOI] [PubMed] [Google Scholar]


