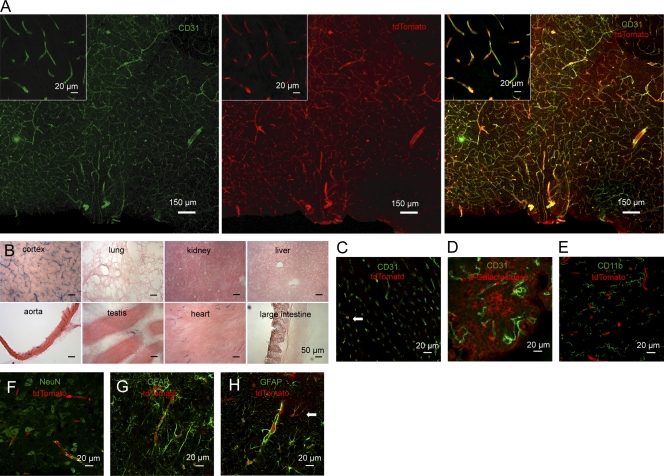
Figure 2.
Brain endothelial-specific recombination in Slco1c1-CreERT2 mice. (A) The preoptic area of Slco1c1-CreERT2; Ai14 mice treated with tamoxifen was subjected to immunostaining with anti-CD31. tdTomato reporter is shown in the middle. Representative stainings from three independent experiments are shown. (B) Histochemical β-Galactosidase staining of several organs of Slco1c1-CreERT2; Rosa26 mice treated with tamoxifen. Representative stainings from three independent experiments are shown. (C) Heart cryosections of tamoxifen-treated Slco1c1-CreERT2; Ai14 reporter mice stained with anti-CD31. 3.2 ± 1.6% of all CD31-positive endothelial cells in the heart were expressing the reporter tdTomato (arrow). Representative stainings from two independent experiments are shown. (D) Immunostaining of brain cryosections of tamoxifen-treated Slco1c1-CreERT2; Rosa26 mice with anti–β-Galactosidase and anti-CD31 to analyze recombination in epithelial and in endothelial cells of the choroid plexus. Representative stainings from two independent experiments are shown. (E–G) Immunofluorescent staining for CD11b, NeuN, and GFAP to determine potential recombination in macrophages/microglia, neurons, and astrocytes in the preoptic area of Slco1c1-CreERT2; Ai14 reporter mice, treated with tamoxifen. Representative stainings from three independent experiments are shown. (H) The cortex of Slco1c1-CreERT2; Ai14 reporter mice treated with tamoxifen was subjected to immunostaining with anti-GFAP. tdTomato showed recombination in endothelial cells surrounded by GFAP-positive astrocytic end-feet. In addition, in the cortex a small number of astrocytes had undergone recombination (arrow). Representative stainings from three independent experiments are shown.