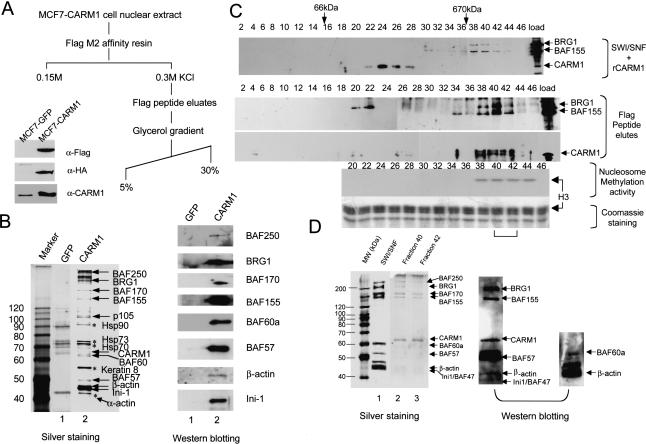
Figure 1.
Characterization of CARM1-complex. (A) Schematic for CARM1-complex purification. Western blot showing detection of Flag-CARM1-HA in transfected MCF7 (lane 2) compared with the control GFP-stable cells (lane 1) using α-flag, HA, or CARM1 antibodies. (B) Determination of CARM1-associated polypeptides. Silver-staining (left) and Western analyses (right) of nuclear proteins from GFP-stable cells (lane 1) and CARM1-stable cells (lane 2) after Flag-peptide elution. Half of the gel was subjected to Western blotting with antibodies against SWI/SNF subunits (right) and superimposed on the silver-stained gel (left) to position each subunit. Heat-shock proteins and α-actin (asterisks) are probably contaminants, as they were also present in control MCF7-GFP cell eluate (lane 1). (C) Western analysis of CARM1, SWI/SNF, and Flag-eluted CARM1 complex by glycerol gradient. Premixed CARM1 and SWI/SNF (top) or Flag-eluted complex (bottom) was applied to a 5%-30% (v/v) gradient. A total of 100 μL of fractions were collected and immunoprecipitated with 20% (w/v) TCA, followed by Western detection with the mixture of BRG1, BAF155, and CARM1 antibodies. BRG1 and BAF155 peaks at fraction 40 are separated from CARM1 peak at fraction 24 (top). The fractions where BSA (66 kD) and Thyroglobulin (670 kD) molecular weight markers sediment are shown on the top of the blot. CARM1 in the Flag-eluted complex cosediments with BRG1 and BAF155 through glycerol gradient (bottom). A total of 10 μL of each Flag-eluted fraction was assayed for methyltransferase activity with 1.5 μg of nucleosomes and 3H-AdoMet as substrates. The autoradiography shows the methylation activity is concentrated in fractions 38-44 and occurs on histone H3 when superimposed on the Coomassie-staining gel. The bracket indicates fractions being further analyzed in D. (D) Silver-staining (left) and Western blotting (right) of NUMAC in fractions 40 and 42 from C. Fractions were TCA precipitated, SDS-PAGE resolved, and silver stained (left) or analyzed by Western blotting (right). The weak staining of small molecular-weight BAFs by silver is probably due to inefficient precipitation by TCA, as the non-TCA-precipitated samples contain small molecular weight BAFs as revealed by Western blotting (right). After Western transfer, the nitrocellulose membranes were first blotted with α-BAF60a/β-actin antibodies, then stripped and reprobed with the mixture of BRG1/BAF155/CARM1/BAF57/INI-1 antibodies.