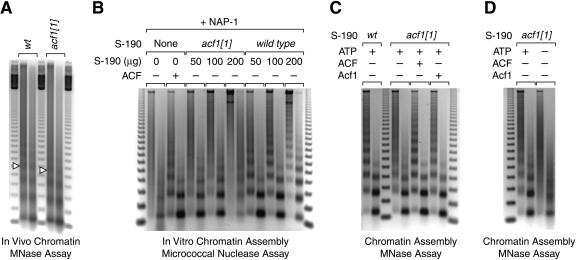
Figure 2.
Mutation of acf1 reduces chromatin assembly activity in embryos. (A) Mutation of acf1 results in reduced nucleosomal periodicity as well as a shorter nucleosome repeat length in Drosophila embryos. Nuclear chromatin from 0- to 12-h embryos was partially digested in situ with micrococcal nuclease by using two different concentrations of enzyme. The resulting partially digested DNA was analyzed by agarose gel electrophoresis and stained with ethidium bromide. The open triangles indicate DNA fragments derived from tetranucleosomes. (B) ACF/CHRAC is the major ATP-dependent chromatin assembly factor in Drosophila S-190 embryo extracts. S-190 extracts were prepared from wild-type and acf1 null embryos. Chromatin was assembled on relaxed plasmid DNA in the presence of the Drosophila core histone chaperone NAP-1. The source of ATP-dependent chromatin assembly activity was either recombinant ACF or the indicated amounts of the S-190 extracts. The reaction products were analyzed by using the micrococcal nuclease digestion assay, in which two different concentrations of the enzyme were used in the analysis of each assembly reaction. (C) ATP-dependent chromatin assembly activity in Acf1-deficient extracts can be restored by biochemical complementation with either purified recombinant ACF complex or purified recombinant Acf1 polypeptide. Chromatin was assembled in the presence of purified recombinant NAP-1 and either wild-type or Acf1-deficient S-190 extract (100 μg). Where indicated, reactions contained purified recombinant ACF or Acf1 (110 fmole). (D) The residual chromatin assembly activity in Acf1-deficient extracts is dependent on ATP. Chromatin was assembled with purified recombinant NAP-1 and Acf1-deficient S-190 extract (100 μg) in the presence or absence of 3 mM ATP (and ATP-regeneration system).