Abstract
Background. We assessed the impact of 12 years of pneumococcal conjugate vaccine (PCV7) use on pneumococcal nasopharyngeal carriage and serotype-specific invasive disease potential among Native Americans.
Methods. Families were enrolled in a carriage study from 2006 to 2008; nasopharyngeal specimens and risk factor information were collected monthly for 7 visits. Pneumococcal carriage prevalence was compared with that before (1998–2000) and during (2001–2002) PCV7 introduction. We compared invasive disease incidence and carriage prevalence before and after PCV7 introduction to estimate changes in serotype-specific invasive potential.
Results. We enrolled 1077 subjects from 302 households. There was an absolute reduction in carriage prevalence of 8.0% (95% confidence interval [CI], 4.5%–11.4%) in children aged <5 years and 3.1% (95% CI, 1.1%–5.1%) in adults. In children aged <5 years, vaccine-serotype carriage prevalence decreased by 22.8% (95% CI, 20.1%–25.3%), and nonvaccine serotype (NVT) increased by 15.9% (95% CI, 12.4%–19.3%). No significant change was detected in serotype-specific invasive potential after PCV7 introduction.
Conclusions. Pneumococcal carriage prevalence decreased in all ages since PCV7 introduction; vaccine-serotype carriage has been nearly eliminated, whereas the prevalence of NVT carriage has increased. The increase in the NVT invasive disease rate seems to be proportional to the increase in colonization prevalence.
Streptococcus pneumoniae (pneumococcus) remains a major cause of pneumonia, meningitis, and sepsis worldwide, especially in young children. The 7-valent pneumococcal polysaccharide conjugate vaccine, PCV7 (Prevnar (Prevenar); Pfizer), contains capsule polysaccharide antigen of 7 serotypes (4, 6B, 9V, 14, 18C, 19F, and 23F). Its routine use among infants has had a significant impact on vaccine serotype (VT) invasive pneumococcal disease (IPD) and nasopharyngeal (NP) carriage among age groups targeted for vaccination and other age groups; however, concomitant increases in IPD and carriage due to serotypes not included in the vaccine (nonvaccine serotypes [NVTs]) have been observed [1–4].
Pneumococcal NP carriage studies conducted during (1998–2000) and immediately after (2001–2002) a community-randomized PCV7 efficacy trial among Navajo and White Mountain Apache children also identified increased carriage with NVTs [5–7]. The increased prevalence of some vaccine-associated serotypes (VATs, a subgroup of NVTs defined as serotypes within the same serogroup as VTs) and other NVT strains since routine use of PCV7 has been temporally associated with observed increases in the rate of NVT IPD in the United States and in other routine-use countries [8–10]. However, the increased incidence of NVT IPD does not tell us whether NVTs have an increased invasive potential or whether the niche left empty by VTs has only given NVTs more opportunity to colonize and therefore more opportunity to cause disease. Invasive potentials of serogroups in the absence of vaccination have been shown to be geographically and temporally stable [11].
The objective of this study was to determine serotype-specific carriage among Navajo and White Mountain Apache children and adults after 8 years of routine PCV7 use and 12 years after initial use in their communities and to assess whether invasive potentials of serotypes have changed in the setting of long-term PCV7 use. These communities are known to have high prevalence of pneumococcal colonization and IPD rates >4-fold that of the general US population [12]. Based on findings from pre-PCV7 studies [11], we hypothesized that there would be no change in the invasive potential of pneumococcal serotypes. Finally, analysis of individual and household characteristics can identify risk factors associated with carriage in this population. Because IPD episodes are preceded by pneumococcal colonization, identifying modifiable carriage risk factors informs additional disease prevention methods.
METHODS
NP Colonization
A prospective, longitudinal, observational cohort study of Navajo and White Mountain Apache families living on reservations in the southwest United States was conducted from March 2006 to March 2008. Parents were approached by research staff during well-child or ill visits at Indian Health Service (IHS) clinics. Families were included if ≥1 parent was a member of the Navajo Nation or White Mountain Apache tribe, the family’s home was on or near the Navajo or White Mountain Apache reservation, ≥1 child in the household was <9 years old, and ≥2 persons in the household would participate in the study for the 6-month time period. Enrolled families were visited monthly for a 6-month period (ie, 7 visits). Nasopharyngeal swab samples and carriage risk factor questionnaires were collected at each visit; household risk factors, including number of children living in the household and the presence of running water, were assessed only at enrollment. We reviewed medical charts of study subjects to assess their pneumococcal immunization record, antibiotic use and hospitalizations (all ages), and outpatient illnesses (children aged <5 years) occurring during the study period.
Nasopharyngeal specimens were obtained with Dacron swabs, using methods described elsewhere [13]. A 100-μL aliquot of each NP specimen in skim milk, tryptone, glucose, and glycerin transport medium was inoculated onto trypticase soy agar with 5% sheep blood and gentamicin (Becton Dickinson); pneumococci were isolated and identified in the Centers for Disease Control and Prevention (CDC) Respiratory Diseases Branch Streptococcus Laboratory, using methods described elsewhere [14]. Serotype 6C was recently identified as antigenically distinct from serotype 6A using monoclonal antibodies [15]. Polymerase chain reaction [14] or a Quellung reaction with specific factor sera [16, 17] was used to distinguish 6A from 6C among carriage isolates originally identified as 6A.
Invasive Pneumococcal Disease
Active, population-based, laboratory-based surveillance for IPD has been conducted on the reservations since the late 1980s, as described elsewhere [12]. Hospital microbiology laboratories serving the population both on the reservations and in surrounding areas are contacted daily to weekly in person or by phone to identify cases among tribal members. IPD isolates were serotyped by the Quellung reaction at the CDC Arctic Investigations Program; all invasive pneumococcal isolates initially serotyped as 6A were tested by polymerase chain reaction to distinguish 6A from 6C at the CDC Respiratory Diseases Branch Streptococcus Laboratory [14].
Statistical Analysis
Nasopharyngeal serotype prevalence was calculated as the number of serotype-specific isolations across all visits divided by the total number of samples collected. To account for correlations between visits, the prevalence was calculated and variance estimated using a linear probability model in Stata 9.1 software [18]. The dependent variable was the count of serotype-specific isolations by study subject, and the distribution of the dependent variable (family) was defined as binomial with an identity link to specify a linear regression. The binomial denominator was indicated as the number of samples collected from each study subject, and the scale parameter was set to the Pearson χ2 statistic for continuous distributions.
We compared overall and serotype-specific pneumococcal carriage prevalence in this study to that described before PCV7 introduction (ie, the control arm of the longitudinal NP study nested within the community randomized PCV7 efficacy trial, 1998–2000) and during early routine use of PCV7 (2001–2002) (a cross-sectional study) using χ2 or Fisher’s exact tests when appropriate. Details of the earlier studies have been published elsewhere [5, 13, 19]. We analyzed potential carriage risk factors using random-effects logistic regression models. Study subject and household were specified as random effects to accommodate for within-subject and within-household correlations. For this 2-level clustering analysis, the software automatically applied an exchangeable correlation structure to the random effects, meaning it assumed the risk factor data collected, by subject or household, was exchangeable across visits. This assumption is valid for the household data because household data did not vary through the study; however, some individual risk factor information did change by visit. Separate models for children and adults in addition to an all-ages model were generated with Stata 9.1 software.
A serotype-specific invasive potential analysis was undertaken, using carriage and IPD serotype data from children aged <5 years. We limited the analysis to this age group, because carriage samples collected in this population before introduction of PCV7 were primarily limited to children aged <5 years [13], and the low carriage prevalence of serotypes important in IPD in adults from the 2001–2002 study prevented our analysis. Serotype data of IPD isolates from children aged <5 years were from the active surveillance of IPD in these communities during the 2 time periods.
The invasive potentials of pneumococcal serotypes before and after PCV7 introduction were calculated as invasiveness rates (InvRs). The formula was defined as follows: InvR = Serotype IPD Incidence/Serotype Carriage Prevalence.
We measured the serotype-specific IPD incidence rate before and after introduction of PCV7 into the community. Annual IHS user population estimates were used as denominators in the calculation of incidence rates [20]. Invasiveness rate of serotypes by era were compared using a test of interaction. The log difference of each serotype’s InvR by era was calculated and divided by the standard error of the log difference to obtain a Z score that was tested for significance; the standard error was used to obtain a 95% confidence interval (CI) for the InvR ratio (InvRR) [21]. Further description is provided in the Supplementary Appendix.
This study was approved by the institutional review boards of the Johns Hopkins Bloomberg School of Public Health, the Navajo Nation, and the Phoenix Area Indian Health Service. Tribal approval was given by the Navajo Nation and the White Mountain Apache tribe. Adults and parents or guardians of children enrolled in the carriage studies provided written informed consent.
RESULTS
Study Subject and Household Characteristics
We enrolled 1077 study subjects (626 children, 451 adults) from 302 households between March 2006 and September 2007, with final specimen collections in March 2008. Seventy-five individuals (7.0%) withdrew during the study period, primarily because the family was leaving the area (n = 27; 2.5%) or because the study subject wanted no further specimens collected (n = 21; 1.9%). A total of 868 (81.0%) study subjects completed ≥6 of 7 study visits, and 657 (61.3%) completed all 7 visits. The median interval between study visits was 30.2 days.
Household and individual characteristics are summarized in Table 1. Notably, >95% of enrolled children aged 2 to <5 years had been vaccinated with ≥3 doses of PCV7. The IHS physicians serving the Navajo Nation follow the US recommendations for routine use of the 23-valent pneumococcal polysaccharide vaccine (PS23) among adults aged ≥65 years and for those aged 2–64 years with specific underlying medical conditions [22]. On the White Mountain Apache reservation, the IHS recommends routine PS23 vaccine administration to begin at 55 years of age. All 7 adults aged ≥65 years enrolled in the study had been previously vaccinated with PS23, as documented in their medical record.
Table 1.
Summary of Interview-Based Household and Individual Characteristics in the Study
| Characteristics | Value |
| Household characteristics | |
| Household members, mean (median), No. | 6 (5) |
| Children aged ≤5 years, mean (median), No. | 2 (2) |
| Total rooms, mean (median), No. | 6 (6) |
| Sleep rooms, mean (median), No. | 3 (3) |
| Running water, No. of households (%) | 250 (83.6) |
| Toilet facilities, No. of households (%) | 245 (81.9) |
| Smoke exposure, No. of households (%) | |
| Wood-burning stove, No. of households (%) | 184 (61.7) |
| Smoker living in home, No. of households (%) | 78 (26.3) |
| Individual characteristics | |
| Subjects by age at enrollment, No. (%) | |
| All subjects | |
| <2 y | 232 (21.6) |
| <2 to <5 y | 227 (21.1) |
| 5 to <9 y | 169 (15.7) |
| 17 to <40 ya | 383 (35.7) |
| 40 to <65 y | 54 (5.0) |
| ≥65 y | 7 (0.7) |
| Breast-fed subjects | |
| <2 | 111 (47.8) |
| 2 to <5 | 77 (33.9) |
| Subjects with episode of OM during study | |
| <2 y | 70 (30.2) |
| 2 to <5 y | 26 (11.5) |
| 5 to <9 y | 11 (6.5) |
| Subjects who used antibiotics during study | |
| <2 y | 77 (33.2) |
| 2 to <5 y | 38 (16.7) |
| 5 to <9 y | 18 (10.7) |
| 17 to <40 y | 49 (12.8) |
| 40 to <65 y | 9 (16.7) |
| ≥65 y | 1 (14.3) |
| Subjects who attended daycare during studyb | |
| <2 y | 9 (3.9) |
| 2 to <5 y | 18 (7.9) |
| 5 to <9 y | 3 (1.8) |
| Daycare attendance per week, mean (median), d | 5 (5) |
| Daycare attendance per week, mean (median), h | 7 (8) |
| Tobacco use (among subjects ≥18 years old), No. (%) | |
| Smoking | 60 (13.5) |
| Chewing | 28 (6.3) |
| Immunization coverage by age at enrollment, No. (%) | |
| PCV7 immunizationc | |
| 1 to <2 y (n = 75) | 71 (94.7) |
| 2 to <5 y (n = 212) | 204 (96.2) |
| 5 to <9 y (n = 150) | 124 (82.7) |
| PS23 immunization coveragec | |
| 17 to <40 y (n = 357) | 17 (4.8) |
| 40 to <65 y (n = 59) | 17 (28.8) |
| ≥65 y (n = 8) | 8 (100.0) |
Abbreviations: OM, otitis media; PCV7, pneumococcal conjugate vaccine; PS23, polysaccharide vaccine.
This group includes 2 parents who were <17 years old.
Daycare was defined as ≥4 h/wk outside the home with ≥2 other children from different households.
Data from chart review, not household interview.
NP Specimens and Carriage Prevalence
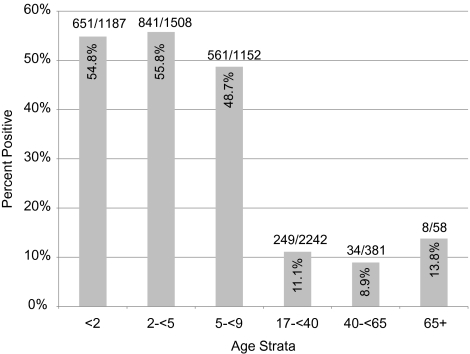
A total of 6541 NP specimens were collected. Of these, 2344 (35.8%) were positive for pneumococcus (Figure 1). Although children aged 5 to <9 years had nearly 50% carriage prevalence, this was significantly lower than carriage among those aged <5 years, for whom prevalence was 55.4% (P < .001). Adults had significantly lower carriage prevalence compared with all children (P < .001). Carriage prevalence significantly differed across calendar months (test of homogeneity, P <.001), with a peak in prevalence December through February (score test, P < .001).
Figure 1.
Number of nasopharyngeal specimens collected and percentage positive for pneumococcus by age strata.
Comparison With Pre-PCV7 Carriage Prevalence
Pneumococcal carriage prevalence in children aged <5 years was 63.4% before PCV7 introduction (1998–2000) [13]. In the routine PCV7 era (2006–2008), the prevalence of pneumococcal carriage in children aged <5 years was 55.4% (Figure 1). Comparing the 2006–2008 data with the 1998–2000 data, the prevalence of pneumococcal carriage decreased by 8.0% (95% CI, 4.5%–11.4%) in absolute terms among children <5 years of age. A stratified analysis of the carriage prevalence comparison by children <2 and 2 to <5 years of age showed no difference compared with the combined analysis. During the early phase of routine vaccine use from February 2001 to January 2002, carriage prevalence among adults was 13.9%, [5] compared with 10.9% in 2006–2008 (Figure 1) representing a 3.1% (95% CI, 1.1%–5.1%) absolute reduction in carriage prevalence. The median ages of adults (31 and 28 years, respectively) and age ranges (18–96 and 17–85 years, respectively) were similar in the 2001–2002 and 2006–2008 studies.
Serotype-Specific Prevalence
Forty-three serotypes were identified among the NP samples in this study (Table 2). Of the 233 isolates originally serotyped as 6A, 228 (97.9%) were found to be 6C. Five serotypes (6C, 19A, 23B, 35B, and 22F) and nontypeable pneumococci were among the top 10 serotypes in all age strata and accounted for 35% of colonizing strains. In contrast, the most common serotype causing IPD in this population, serotype 12F [12], was not among the top 10 colonizing strains. The 10 serotypes most detected in carriage within different age groups are listed in Table 3. Serotype carriage distributions were similar between age groups with a few exceptions. Serotypes 15C and 23A were among the top 10 serotypes for children aged <2 years, and serotypes 16F and 21 were in the top 10 only for those aged 2 to <5 years. Serotypes 3 and 10A were in the top 10 only for children aged 5 to <9 years, but serotypes 17F and 34 shared top 10 status with both children aged 5 to <9 years and adults. Serotype 7C was in the top 10 serotypes only for adults.
Table 2.
Serotype Prevalence in NP Specimens Obtained From All Study Subjects Enrolled in the Study (N = 6541)
| Serotype | Serotype, No. | Prevalence, % | 95% CI, %a |
| Vaccine types (VTs) | |||
| All VTs | 56 | 0.9 | .6–1.1 |
| 19F | 35 | 0.5 | .3–.8 |
| 9V | 7 | 0.1 | .0–.2 |
| 4 | 5 | 0.1 | .0–.2 |
| 14 | 4 | 0.1 | .0–.1 |
| 6B | 4 | 0.1 | .0–.2 |
| 18C | 1 | 0.02 | .0–.04 |
| All NVTs | 2357 | 36.0 | 34.9–37.3 |
| Vaccine-associated types (VATs) | |||
| All VATs | 676 | 10.3 | 9.5–11.1 |
| 6Cb | 228 | 3.5 | 3.1–4.0 |
| 19A | 208 | 3.2 | 2.5–3.8 |
| 23B | 137 | 2.1 | 1.6–2.6 |
| 23A | 89 | 1.4 | 1.0–1.8 |
| 9N | 9 | 0.1 | .1–.2 |
| 6A | 5 | 0.1 | .0–.2 |
| NVTs excluding VATs | |||
| 35B | 137 | 2.1 | 1.8–2.5 |
| 22F | 134 | 2.1 | 1.7–2.4 |
| NT | 133 | 2.0 | 1.7–2.4 |
| 15A | 132 | 2.0 | 1.7–2.4 |
| 17F | 96 | 1.5 | 1.0–1.9 |
| 11A | 92 | 1.4 | 1.0–1.9 |
| 34 | 89 | 1.4 | 1.0–1.7 |
| 21 | 88 | 1.3 | .9–1.8 |
| 10A | 85 | 1.3 | .9–1.7 |
| 16F | 83 | 1.3 | .1–1.7 |
| 15B | 74 | 1.1 | .9–1.4 |
| 3 | 73 | 1.1 | .9–1.4 |
| 33F | 60 | 0.92 | .6–1.2 |
| 7C | 60 | 0.92 | .6–1.2 |
| 35F | 58 | 0.89 | .6–1.2 |
| 35A | 55 | 0.84 | .6–1.2 |
| 15C | 50 | 0.76 | .5–1.0 |
| 31 | 50 | 0.76 | .5–1.0 |
| 38 | 32 | 0.49 | .3–.7 |
| 12F | 25 | 0.38 | .2–.6 |
| 10F | 25 | 0.38 | .2–.6 |
| 7F | 13 | 0.20 | .1–.3 |
| 8 | 11 | 0.17 | .0–.3 |
| 37 | 10 | 0.15 | .0–.3 |
| 1 | 8 | 0.12 | .0–.2 |
| 5 | 2 | 0.03 | .0–.1 |
| 10C | 1 | 0.02 | .0–.1 |
| 20 | 1 | 0.02 | .0–.1 |
| 22A | 1 | 0.02 | .0–.1 |
| 27 | 1 | 0.02 | .0–.1 |
| 28 | 1 | 0.02 | .0–.1 |
| 33A | 1 | 0.02 | .0–.1 |
If 95% confidence interval (CI) crossed 0 (owing to extrabinomial variation common with identity link models), .0 is listed as the low end of interval.
This group includes 3 isolates that were not available for retesting and were assumed to be 6C.
Table 3.
Distribution of 10 Most Frequently Isolated Serotypes From Carriage Swab Samples Positive for Pneumococcus Obtained From Children and Adults Enrolled in the 2006–2008 Study
| Top 10 Serotypes by Age Group | Samples, No. | Proportion, % |
| Children aged <2 y (n = 669) | ||
| 6C | 78 | 11.7 |
| 19A | 69 | 10.3 |
| 15A | 46 | 6.7 |
| 23B | 44 | 6.6 |
| 35B | 39 | 5.8 |
| 22F | 34 | 5.1 |
| 23A | 30 | 4.5 |
| NT | 27 | 4.0 |
| 11A | 26 | 3.9 |
| 15C | 23 | 3.4 |
| Children aged 2 to <5 y (n = 867) | ||
| 6C | 77 | 8.9 |
| 19A | 73 | 8.4 |
| 15A | 55 | 6.3 |
| 22F | 55 | 6.3 |
| NT | 45 | 5.2 |
| 23B | 44 | 5.1 |
| 35B | 44 | 5.1 |
| 11A | 41 | 4.7 |
| 16F | 38 | 4.4 |
| 21 | 37 | 4.3 |
| Children aged 5 to <9 y (n = 586) | ||
| 6C | 45 | 7.7 |
| 19A | 41 | 7.0 |
| 23B | 37 | 6.3 |
| NT | 35 | 6.0 |
| 35B | 34 | 5.8 |
| 22F | 29 | 5.0 |
| 3 | 28 | 4.8 |
| 17F | 27 | 4.6 |
| 10A | 25 | 4.3 |
| 34 | 24 | 4.1 |
| Adults (≥17 y) (n = 288) | ||
| 6C | 28 | 9.7 |
| 19A | 25 | 8.7 |
| NT | 25 | 8.7 |
| 35B | 20 | 6.9 |
| 7C | 20 | 6.9 |
| 34 | 19 | 6.6 |
| 17F | 18 | 6.3 |
| 22F | 16 | 5.6 |
| 15A | 12 | 4.2 |
| 23B | 10 | 3.5 |
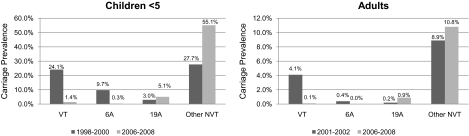
Comparing the carriage serotype prevalence data among NP specimens from children aged <5 years with data from the pre- and early PCV7 era [13], VT carriage prevalence among collected specimens decreased in absolute terms by 22.7% (95% CI, 20.1%–25.3%) from 24.1% to 1.4% (Figure 2). The overall prevalence of the VAT subset of the NVT strains did not change significantly in the 2006–2008 study compared with the pre-PCV7 era (14.5% and 14.6%, respectively); however, within the VAT subset, serotype 6A decreased from 9.7% (estimate from published subanalysis of conventionally serotyped 6A isolates [14]) to 0.3% prevalence (P < .01). The NVT carriage prevalence (excluding 6A) among collected specimens increased in absolute terms by 15.9% (95% CI, 12.4%–19.3%), from 27.7% to 55.1%. Trends were identical for adults and children (Figure 2).
Figure 2.
Comparison of absolute carriage prevalence by type in children aged <5 years (years 1998–2000 vs 2006–2008) and in adults (years 2001–2002 vs 2006–2008). NVT, nonvaccine serotype; VT, vaccine serotype.
Pneumococcal Invasive Potential
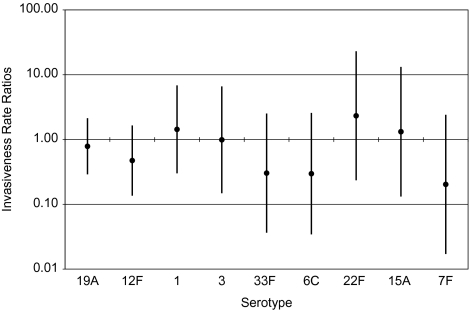
We assessed changes in invasive potential of the serotypes causing IPD episodes in the PCV7 era [12], including serotypes 19A (9 episodes), 12F (7 episodes), 1 (7 episodes), 3 (3 episodes), 33F (2 episodes), 6C (1 episode), 22F (3 episodes), 15A (3 episodes), and 7F (4 episodes) (Figure 3). Consistent with our expectation, all InvRRs had CIs that spanned 1, meaning we were unable to detect changes in invasive potentials of NVTs between the pre-and routine PCV7 eras. However, our level of certainty about the similarity of invasive potentials across eras varied by serotype. Although broad, the 95% CIs on the InvRRs for serotypes 19A, 12F, 1, and 3 suggest that the difference in invasive potentials between the two eras was no more than 10-fold. For other serotypes, the uncertainty range was even broader because of low prevalence in either colonization or disease resulting in limited precision.
Figure 3.
Invasiveness rate ratios by serotype, sorted by increasing variance.
Carriage Risk Factors
Risk factor analyses for pneumococcal carriage are summarized in Table 4. In the all-ages model, study subjects living in a household with a colonized child aged <9 years were at increased risk of colonization (odds ratio [OR], 3.15; 95% CI, 2.60–3.83). Antibiotic use reported during a visit was associated with a decreased risk of colonization at that visit (OR, 0.40; 95% CI, .27–.59). Increasing age was associated with a decreased risk of colonization (OR, 0.92/y; 95% CI, .91–.92). Being male was associated with an increased risk of carriage (OR, 1.29; 95% CI, 1.03–1.61), which has been described elsewhere in this population [13].
Table 4.
Risk of Pneumococcal Carriage From Household (HH) and Individual Carriage Analysis by All Ages, Children Aged <9 Years, and Adults Aged ≥17 Years
| Carriage Risk Factors | Odds Ratio | 95% CI | P |
| All ages | |||
| Age | 0.9 | .91–.92 | <.001 |
| Male sex | 1.3 | 1.03–1.61 | .029 |
| Antibiotic use | 0.4 | .27–.59 | .000 |
| No. of HH members | 1.0 | .95–1.13 | .383 |
| No. of children aged <5 y in HH | 1.3 | 1.11–1.54 | .001 |
| Child aged <9 y colonizeda | 3.2 | 2.60–3.83 | <.001 |
| Running water | 0.7 | .49–1.09 | .119 |
| Wood-burning stove | 1.2 | .86–1.61 | .301 |
| Rooms for sleep | 0.9 | .78–1.05 | .173 |
| Smoker in house | 1.1 | .78–1.52 | .637 |
| Children aged <9 y | |||
| Age | 0.9 | .83–.93 | <.001 |
| Male sex | 1.1 | .81–1.35 | .713 |
| Antibiotic use | 0.4 | .23–.54 | .000 |
| Currently breast-feeding | 1.2 | .78–1.70 | .469 |
| Daycare utilization | 1.0 | .51–2.14 | .912 |
| No. of HH members | 1.0 | .97–1.11 | .242 |
| ≥3 doses of PCV7 | 0.9 | .61–1.44 | .774 |
| Child aged <9 y colonizeda | 3.6 | 2.90–4.51 | <.001 |
| Running water | 1.1 | .75–1.60 | .628 |
| Wood-burning stove | 1.5 | 1.10–1.95 | .009 |
| Rooms for sleep | 0.9 | .80–1.05 | .231 |
| Adults | |||
| Age | 1.0 | .97–1.02 | .537 |
| Male sex | 1.2 | .71–1.90 | .559 |
| Antibiotic use | 0.3 | .06–1.28 | .102 |
| No. of HH members | 1.0 | .86–1.11 | .713 |
| No. of children aged <5 y in HH | 1.4 | 1.10–1.81 | .006 |
| Any past receipt of PS23 | 1.4 | .66–3.01 | .374 |
| Child aged <9 y colonizeda | 2.0 | 1.35–3.01 | .001 |
| Running water | 0.5 | .31–.92 | .025 |
| Rooms for sleep | 0.9 | .73–1.13 | .383 |
Abbreviations: CI, confidence interval; PCV7, pneumococcal conjugate vaccine; PS23, polysaccharide vaccine.
Exposure to a colonized child aged <9 years.
In a separate analysis including only data from children <9 years old, age and living with a colonized child aged <9 years old continued to be significantly associated with colonization. Antibiotic use was associated with a reduced risk of colonization, and the presence of a wood-burning stove was associated with an increased risk of colonization in children aged <9 years (OR, 1.46; 95% CI, 1.10–1.95). Sex, current breast-feeding, daycare utilization, ≥3 doses of PCV7, the presence of running water, and rooms for sleeping were not significantly associated with colonization in children. In adults, the presence of a colonized child aged <9 years in the household continued to be a risk factor for carriage (OR, 2.02; 95% CI, 1.35–3.01). The presence of running water in the household was significantly associated with a decreased risk of colonization (OR, 0.53; 95% CI, .31–.92), suggesting that hygiene may play a role in preventing pneumococcal carriage among adults.
DISCUSSION
Long-term routine use of PCV7 has resulted in near elimination of VT and vaccine-associated serotype 6A pneumococcal carriage among Navajo and White Mountain Apache people of all ages. Although we observed an increase in the prevalence of carriage with NVTs in children aged <5 years, the overall reduction in VT carriage prevalence was greater than the NVT increase, resulting in a significant decrease in overall pneumococcal carriage. This decrease is in contrast to observations in other US populations, in which NVTs often fully replaced VTs [14]. Hypotheses to explain this difference include replacement with nonpneumococcal species (such as Staphylococcus aureus), improvements in environmental risk factors for pneumococcus, and lower rates of antibiotic resistance among pneumococci in the Navajo and White Mountain Apache people, thereby reducing the selective pressure for resistant pneumococci. Regardless, carriage prevalence remains higher in these populations than in other US populations.
Although the invasive potential analysis was limited in power, we found no evidence that serotype-specific invasive potentials have changed. Because PCV7 serotypes were chosen for inclusion in the vaccine based on their prevalence in IPD, we do not expect to see complete replacement in IPD by NVTs in the general US population. However, among Navajo people, the proportion of IPD caused by NVT strains before vaccine introduction was higher than in the general US population [23]. Therefore, the percentage reduction in overall IPD among Navajo and White Mountain Apache people of all ages in the PCV7 era (24%; 95% CI, 12%–40%)] [12] is less than what is observed in the general US population (45%; 95% CI, 42%–47%) [2]. Thus, although absolute rates of IPD have decreased in these Native American communities in the PCV7 era, the relative disparity in IPD rates compared with the remaining IPD in the general US population has actually increased (2.7 vs 3.8 times greater).
The risk factor analysis continues to support the hypotheses that age, exposure to other colonized children, and wood-burning stoves are associated with an increased risk of carriage in children. As expected, antibiotic use temporarily decreases pneumococcal carriage prevalence. This protection was not observed in adults (Table 4). It is possible that antibiotics prescribed more often to adults do not eliminate pneumococcal carriage. Active breast-feeding did not appear to play a protective role in pneumococcal carriage in children. Antibodies passively transferred by breast-feeding may provide protection to an infant from IPD [24], but the type and target of the antibodies may not protect against carriage. We were unable to find an association between daycare attendance and pneumococcal carriage, but daycare utilization was low, with only 4% of children aged <2 years and 8% of children aged 2 to <5 years attending daycare at any point during the study period. Exposure to colonized children in the household was a significant risk factor for carriage among adults. The presence of running water in the household was associated with protection against carriage in adults but not in children. It is possible that because hygienic practices are an acquired behavior, adults are more likely to use these practices when running water is available. The presence of running water is indicative of a higher household socioeconomic status. These adults may spend more time outside the home and may not be regularly and continuously exposed to pneumococci carried by other household members.
There are several limitations to this study. Risk factor data could be subject to reporting bias, particularly for more sensitive data such as smoking or breast-feeding. Serotypes of short carriage duration are less likely to be identified with monthly sampling than with a more frequent sampling period; thus, our observed serotype distribution is biased toward those serotypes with longer duration of colonization. The isolation techniques potentially missed types that are not easily cultured and undoubtedly missed some strains that were present in low density or that comprised smaller proportions of total isolates when multiple serotypes were present [25]. Finally, although randomized trials show that PCV7 vaccination reduces VT carriage and increases NVT carriage in vaccinated individuals [26], and in theory these changes would be amplified in highly vaccinated populations [27], we cannot attribute all changes in carriage and serotype prevalence to PCV7 use; they may also reflect secular trends not related to the vaccine.
This study has shown that long-term routine use of PCV7 among Navajo and White Mountain Apaches coincided with near elimination of VT carriage throughout the community and provides significant cross-protection against serotype 6A. We were not able to detect meaningful changes in the invasive potential of the remaining non-PCV7 serotypes; stable invasive potentials allow for modeling and predictions of serotypes likely to be of future importance in IPD by conducting NP studies. Fifteen percent of all serotypes identified among the carriage isolates from this study are included in PCV13, the pneumococcal conjugate vaccine formulation introduced for routine use in the United States in early 2010. As PCV13 is introduced into the population, we expect to see a continued reduction in carriage of serotypes included in the vaccine among all age groups, as well as a reduction in serotype 6C carriage prevalence should cross-protection be conferred by the 6A polysaccharides in the PCV13 product, as predicted [28]. However, we also expect an increase in carriage prevalence of serotypes not included in PCV13. Based on serotypes prevalent in carriage across all ages, we expect that serotypes 15A, 23A, 23B, 35B, and 22F will probably be of ongoing importance in these populations. Continued monitoring of carriage and IPD will be necessary to determine important serotypes in the PCV13 era. Although the invasive potential analysis suggests that the increase observed in serotype-specific IPD is proportional to the increase observed in serotype-specific carriage prevalence, it is important to continue monitoring these trends. Ultimately, vaccine candidates that provide cross-protection across all serotypes would be optimal for the control of pneumococcal diseases.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://www.oxfordjournals.org/our_journals/jid/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments.
The authors express their appreciation to the children and adults from the Navajo and White Mountain Apache communities who participated actively in the studies. We also gratefully acknowledge the dedicated efforts of the Center for American Indian Health field staff who collected these data over many years, particularly Stella Cly, Stevie Cosay, Virgina Crocker, Beverly Gorman, Lora Lavender, Lori Samuel, and Roxy Thompson. We thank the CDC Respiratory Diseases Branch Streptococcus Laboratory for collaboration on the carriage study; specifically we are grateful for pneumococcal isolation and serotyping by Dee Jackson, NP specimen storage and pneumococcal isolate storage, and the serotyping using newly developed factor sera to resolve past 6A/6C isolates provided by Logan K. Sherwood. We also gratefully acknowledge the serotyping of the invasive isolates by Karen Rudolph and Marcella Harker-Jones at the CDC Arctic Investigations Program, Anchorage, Alaska.
Financial support.
This study is part of the research of the PneumoCarr Consortium funded by the Grand Challenges in Global Health initiative, which is supported by the Bill & Melinda Gates Foundation; the Foundation for the National Institutes of Health; the Wellcome Trust; and the Canadian Institutes of Health Research. This study was supported by The Native American Research Centers for Health (grant U26IHS300013/03), a joint initiative between the National Institutes of Health and Indian Health Service to reduce Native American health disparities and build tribal autonomy in conducting health research. This study was also funded by the Centers for Disease Control and Prevention National Vaccine Program Office and the Thrasher Research Fund.
Potential conflicts of interest.
K. L. O. and M. S. have received grant support and/or honoraria from Wyeth Vaccines (now Pfizer), Sanofi-Pasteur, and Merck. M. L. has accepted consulting fees and advisory meeting honoraria from Novartis and Pfizer for work performed on matters not related to S. pneumoniae. L. H. M. has received honoraria for DSMB and/or advisory boards for Merck, Pfizer, and Novartis. K. L. O. and L. H. M. serve on a Streptococcus pneumoniae vaccine advisory board for Merck. All other authors: no conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Whitney CG, Farley MM, Hadler J, et al. Decline in invasive pneumococcal disease after the introduction of protein-polysaccharide conjugate vaccine. N Engl J Med. 2003;348:1737–46. doi: 10.1056/NEJMoa022823. [DOI] [PubMed] [Google Scholar]
- 2.Pilishvili T, Lexau C, Farley MM, et al. Sustained reductions in invasive pneumococcal disease in the era of conjugate vaccine. J Infect Dis. 2010;201:32–41. doi: 10.1086/648593. [DOI] [PubMed] [Google Scholar]
- 3.Huang SS, Hinrichsen VL, Stevenson AE, et al. Continued impact of pneumococcal conjugate vaccine on carriage in young children. Pediatrics. 2009;124:e1–11. doi: 10.1542/peds.2008-3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Park SY, Moore MR, Bruden DL, et al. Impact of conjugate vaccine on transmission of antimicrobial-resistant Streptococcus pneumoniae among Alaskan children. Pediatr Infect Dis J. 2008;27:335–40. doi: 10.1097/INF.0b013e318161434d. [DOI] [PubMed] [Google Scholar]
- 5.Millar EV, Watt JP, Bronsdon MA, et al. Indirect effect of 7-valent pneumococcal conjugate vaccine on pneumococcal colonization among unvaccinated household members. Clin Infect Dis. 2008;47:989–96. doi: 10.1086/591966. [DOI] [PubMed] [Google Scholar]
- 6.Millar EV, O'Brien KL, Watt JP, et al. Effect of community-wide conjugate pneumococcal vaccine use in infancy on nasopharyngeal carriage through 3 years of age: a cross-sectional study in a high-risk population. Clin Infect Dis. 2006;43:8–15. doi: 10.1086/504802. [DOI] [PubMed] [Google Scholar]
- 7.Millar EV, O'Brien KL, Scott JR, et al. Near elimination of vaccine-type pneumococcal carriage by pneumococcal conjugate vaccine in a community at high risk of carriage and disease. In: Abstracts of the 6th International Symposium on Pneumococci and Pneumococcal Diseases (Reykjavik, Iceland). Congress Reykjavik, 2008. [Google Scholar]
- 8.Hicks LA, Harrison LH, Flannery B, et al. Incidence of pneumococcal disease due to non-pneumococcal conjugate vaccine (PCV7) serotypes in the United States during the era of widespread PCV7 vaccination, 1998–2004. J Infect Dis. 2007;196:1346–54. doi: 10.1086/521626. [DOI] [PubMed] [Google Scholar]
- 9.Kaplan SL, Mason EO, Jr, Wald ER, et al. Decrease of invasive pneumococcal infections in children among 8 children's hospitals in the United States after the introduction of the 7-valent pneumococcal conjugate vaccine. Pediatrics. 2004;113:443–9. doi: 10.1542/peds.113.3.443. [DOI] [PubMed] [Google Scholar]
- 10.Messina AF, Katz-Gaynor K, Barton T, et al. Impact of the pneumococcal conjugate vaccine on serotype distribution and antimicrobial resistance of invasive Streptococcus pneumoniae isolates in Dallas, TX, children from 1999 through 2005. Pediatr Infect Dis J. 2007;26:461–7. doi: 10.1097/INF.0b013e31805cdbeb. [DOI] [PubMed] [Google Scholar]
- 11.Brueggemann AB, Peto TE, Crook DW, Butler JC, Kristinsson KG, Spratt BG. Temporal and geographic stability of the serogroup-specific invasive disease potential of Streptococcus pneumoniae in children. J Infect Dis. 2004;190:1203–11. doi: 10.1086/423820. [DOI] [PubMed] [Google Scholar]
- 12.Weatherholtz R, Millar EV, Moulton LH, et al. Invasive pneumococcal disease a decade after pneumococcal conjugate vaccine use in an American Indian population at high risk for disease. Clin Infect Dis. 2010;50:1238–46. doi: 10.1086/651680. [DOI] [PubMed] [Google Scholar]
- 13.Millar EV, O'Brien KL, Zell ER, Bronsdon MA, Reid R, Santosham M. Nasopharyngeal carriage of Streptococcus pneumoniae in Navajo and White Mountain Apache children before the introduction of pneumococcal conjugate vaccine. Pediatr Infect Dis J. 2009;28:711–16. doi: 10.1097/INF.0b013e3181a06303. [DOI] [PubMed] [Google Scholar]
- 14.Millar EV, Pimenta FC, Roundtree A, et al. Pre- and post-conjugate vaccine epidemiology of pneumococcal serotype 6C invasive disease and carriage within Navajo and White Mountain Apache communities. Clin Infect Dis. 2010;51:1258–65. doi: 10.1086/657070. [DOI] [PubMed] [Google Scholar]
- 15.Park IH, Pritchard DG, Cartee R, Brandao A, Brandileone MC, Nahm MH. Discovery of a new capsular serotype (6C) within serogroup 6 of Streptococcus pneumoniae. J Clin Microbiol. 2007;45:1225–33. doi: 10.1128/JCM.02199-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mercado E, Srinivasan V, Hawkins P, et al. First report of Streptococcus pneumoniae serotype 6D in South America. J Clin Microbiol. 2011;49:2080–1. doi: 10.1128/JCM.00153-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Melnick N, Thompson TA, Beall BW. Serotype-specific typing antisera for pneumococcal serogroup 6 serotypes 6A, 6B, and 6C. J Clin Microbiol. 2010;48:2311–2. doi: 10.1128/JCM.00410-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McCullagh P, Nelder JA. Generalized linear models. 2nd ed. New York: Chapman and Hall; 1989. [Google Scholar]
- 19.O'Brien KL, Moulton LH, Reid R, et al. Efficacy and safety of seven-valent conjugate pneumococcal vaccine in American Indian children: group randomised trial. Lancet. 2003;362:355–61. doi: 10.1016/S0140-6736(03)14022-6. [DOI] [PubMed] [Google Scholar]
- 20. Indian Health Service. Indian Health Service user population statistics. Indian Health Service, Department of Health and Human Services 2008.
- 21.Altman DG, Bland JM. Interaction revisited: the difference between two estimates. BMJ. 2003;326:219. doi: 10.1136/bmj.326.7382.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Advisory Committee on Immunization Practices. Preventing pneumococcal disease among infants and young children: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2000;49:1–35. [PubMed] [Google Scholar]
- 23.O'Brien KL, Shaw J, Weatherholtz R, et al. Epidemiology of invasive Streptococcus pneumoniae among Navajo children in the era before use of conjugate pneumococcal vaccines, 1989–1996. Am J Epidemiol. 2004;160:270–8. doi: 10.1093/aje/kwh191. [DOI] [PubMed] [Google Scholar]
- 24.Levine OS, Farley M, Harrison LH, Lefkowitz L, McGeer A, Schwartz B. Risk factors for invasive pneumococcal disease in children: a population-based case-control study in North America. Pediatrics. 1999;103:E28. doi: 10.1542/peds.103.3.e28. [DOI] [PubMed] [Google Scholar]
- 25.da Gloria Carvalho M, Pimenta FC, Jackson D, et al. Revisiting pneumococcal carriage by use of broth enrichment and PCR techniques for enhanced detection of carriage and serotypes. J Clin Microbiol. 2010;48:1611–8. doi: 10.1128/JCM.02243-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O'Brien KL, Millar EV, Zell ER, et al. Effect of pneumococcal conjugate vaccine on nasopharyngeal colonization among immunized and unimmunized children in a community-randomized trial. J Infect Dis. 2007;196:1211–20. doi: 10.1086/521833. [DOI] [PubMed] [Google Scholar]
- 27.Lipsitch M. Bacterial vaccines and serotype replacement: Lessons from Haemophilus influenzae and prospects for Streptococcus pneumoniae. Emerg Infect Dis. 1999;5:336–45. doi: 10.3201/eid0503.990304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cooper D, Yu X, Sidhu M, Nahm MH, Fernsten P, Jansen KU. In: Abstracts of the 7th International Symposium on Pneumococci and Pneumococcal Diseases (Tel Aviv, Israel) 13-valent pneumococcal conjugate vaccine elicits strong opsonophagocytic killing (OPA) activity to Streptococcus pneumoniae serotypes 6A, 6B, and 6C. Kenes International, 2010. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.