Figure 6.
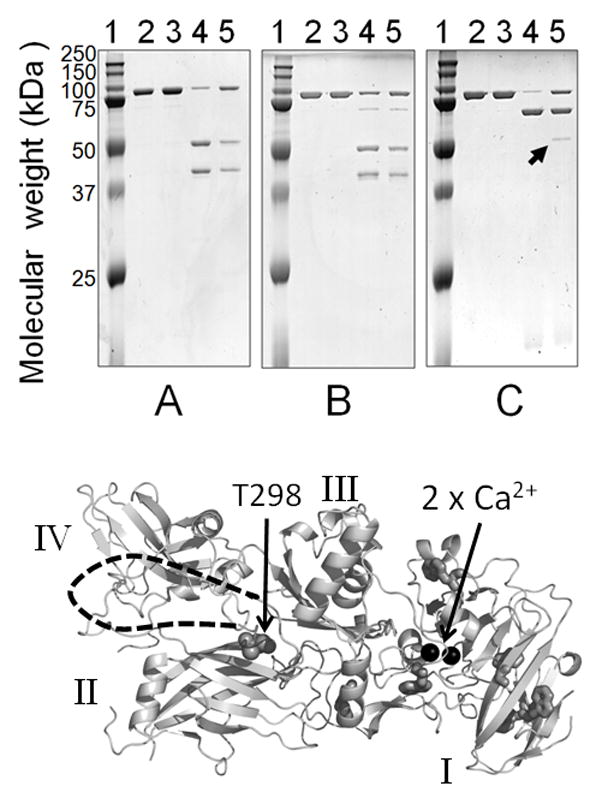
Limited proteolysis of rPA83, Upper –SDS PAGE showing cleavage patterns A) Chymotrypsin, B) Pronase E and C) Trypsin; indicated by the arrow is an additional band produced by trypsin in adsorbed rPA83. Lane 1 = protein standards, lane 2 = rPA83, lane 3 = adsorbed rPA83, lane 4 = soluble rPA83 + protease, and lane 5 = adsorbed rPA83 + protease. Lower- structure of PA82 (PDB;1ACC). Additional cleavage site marked at T296 with unresolved loop indicated by dashed line as in [20]. Also shown are the calcium ions as black spheres, tryptophan residues as sticks and domain structure in roman numerals.
