Abstract
Background
Blood pumps used for temporary circulatory support have limitations. We propose a novel device designed for short-term extracorporeal support which is intrinsically volume responsive, afterload insensitive, and incapable of cavitation or excessive hemolysis. After in vitro testing, we performed the initial in vivo implantations and assessments.
Methods
The BioVAD prototype (MC3, Inc, Ann Arbor, MI) was implanted in six adult male sheep (60.2 +/− 2.8 kg) via the left ventricular apex and descending thoracic aorta. Arterial, left and right atrial, pump inlet and outlet pressures and BioVAD flow were measured and recorded. The animals were volume loaded to assess volume responsiveness, and the inlet lines were abruptly clamped during maximum support to observe for cavitation. An acute heart failure model was created with rapid ventricular pacing, and the animals were supported for 4 hours.
Results
Peak flow was 3.19 +/− 0.56 L/min, and increased with 20 mmHg vacuum assisted drainage to 3.71 +/− 0.53 L/min. Without manual changes in pump settings, pump flow increased 17.5% with volume loading. During acute venous line occlusion, there was no evidence of cavitation and inlet suction was minimal. Hemodynamics were maintained for 4 hours during acute heart failure.
Conclusions
The BioVAD provided adequate flow in an acute in vivo model. Its design may be superior for short-term extracorporeal support.
Keywords: ECMO, Circulatory Assist Device, Artificial Organs
Introduction
Temporary circulatory support, in the form of extracorporeal ventricular assist devices1–3 (VADs) or extracorporeal membrane oxygenation4 (ECMO), is increasingly utilized in the treatment of cardiogenic shock. The mechanical blood pumps employed for these purposes have limitations. Occlusive roller type pumps produce extreme negative or positive pressure during unexpected line obstruction, risking circuit rupture, hemolysis or cavitation. Roller pumps must be continuously observed by trained personnel. Centrifugal design pumps operate at a fixed rotational speed and in some settings may not intrinsically respond to a patient’s changing volume and hemodynamic status. In addition, centrifugal pumps produce unregulated inlet suction, hazarding cavitation and hemolysis. They are also afterload sensitive, generating lower flow in the setting of increased outflow resistance. Pneumatic pulsatile pumps intrinsically respond in a dynamic physiologic environment, but can be thrombogenic, particularly in the sinus regions of the prosthetic valves. An ideal pump for extracorporeal support should be simple, safe, afterload insensitive, responsive to changing preload, biocompatible, and applicable for left (LVAD), right (RVAD), or biventricular support and ECMO. A novel pump designed to satisfy these requirements was previously tested in vitro and favorably compared to predicate devices in terms of hemodynamic profile, durability and hemolysis5. We will now perform the initial in vivo assessment in a large animal model.
Material and Methods
BioVAD
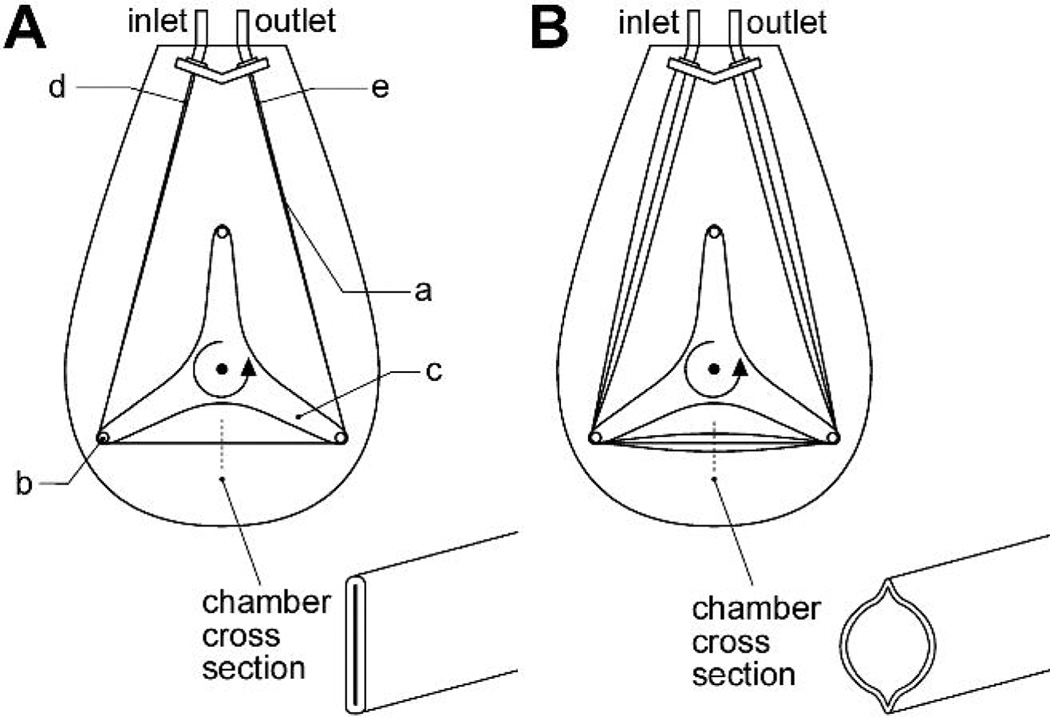
The BioVAD (MC3 Inc, Ann Arbor, MI) is a nonocclusive peristaltic type pump (Figure 1). The device consists of a collapsible polyurethane conduit (the pump chamber) wrapped under tension around three freely rotating rollers. When venous return allows, the pump chamber is full, and blood is ejected by the rollers in peristaltic fashion. If venous return becomes obstructed, the rollers continue to spin, but the pump chamber collapses, eliminating the risk of excessive suction which can cause hemolysis or cavitation. At any degree of venous return between complete pump chamber collapse and full filling, the pump acts in a Starling fashion, intrinsically altering output with varying preload. The pump housing is sealed, allowing vacuum assisted drainage. The BioVAD prototype (Figure 2) has a rotor diameter of 4 inches and the pump chamber has a maximum volume of 65 ml. The pump typically operates between 50 and 100 RPM.
Figure 1.
BioVAD schematic. a: pump chamber; a: roller; c: rotor; d: pump chamber inlet region; e: pump chamber outlet region. Panel A is under conditions of absent venous return, where the pump chamber is flat (inlet pressure=0). Panel B is under conditions of sufficient venous return with expansion of the pump chamber (inlet pressure >0).
Figure 2.
BioVAD prototype (Photo provided by MC3, Inc).
Instrumentation
Animals received humane care in compliance with the National Society of Medical Research and the Institute of Laboratory Animal Resources. The University of Michigan Committee on the Use and Care of Animals, protocol number 10309 approved all experiments. Six adult male sheep, 60.2 +/− 2.8 kg, were anesthetized using sodium thiopental induction (7 mg/kg) and maintained on inhalational anesthesia with Isoflurane 0.1–4%. An infusion of norepinephrine was initiated to maintain stable arterial blood pressure. The dose of the infusion was not adjusted after initiation of BioVAD support. Isotonic saline was infused at approximately 100 ml/hour throughout the conduct of the experiment to account for insensible fluid losses.
A left thoracotomy was performed and the pericardium opened. Heparin was administered (100 IU/kg), and an activated clotting time of >200 seconds was confirmed. A 10mm woven polyester graft bonded to 3/8th inch tubing was anastomosed end to side to the descending thoracic aorta. A 32 French beveled cannula was inserted into the left ventricular apex and controlled with purse string sutures. Using standard connectors, the cannulae were attached to the BioVAD circuit, previously primed with isotonic saline.
Pressure monitoring catheters were placed in the jugular vein, carotid artery, and left atrium. Fluid coupled strain gauge transducers were connected to these catheters as well as to the BioVAD inlet and outline cannulae for continuous pressure measurement and recording. An ultrasonic flow probe was placed on the BioVAD circuit. Pressure and flow measurements were collected continuously at 250 Hz using a standard circuit board and stored on a computer using LabVIEW software (National Instruments, Austin, TX).
Study protocol
After instrumentation and cannula insertion, BioVAD rotational speed was gradually increased until additional adjustments produced little increase in the achieved flow rate. This estimated the maximum unassisted flow for that animal. Vacuum was then incrementally applied to the pump housing to augment drainage, up to a maximum of −20 mmHg. BioVAD vacuum was discontinued and RPM settings were then adjusted to achieve a target flow rate of approximately 50 ml/kg/min and ensuring that the pump chamber was partially filled, and thereby operating in a Starling fashion. These settings were then maintained constant over the subsequent maneuvers. Volume responsiveness was assessed by rapidly infusing one liter of isotonic saline over 1−2 minutes. Once a stable hemodynamic profile was achieved, 500 ml of blood was phlebotomized over 5–10 minutes. This blood was then slowly re-infused. Afterload sensitivity was assessed by administering 200–400 mcg of phenylephrine as a rapid bolus to achieve a mean arterial pressure exceeding 120 mmHg. After subsequent normalization of arterial blood pressure and reestablishment of baseline hemodynamic conditions, the BioVAD inflow line was abruptly clamped, and the circuit was observed and filmed for signs of cavitation. The clamp was removed and after stable support was reached, the outflow line was abruptly clamped and the circuit was observed for disruption or BioVAD malfunction. After steady state was achieved, acute heart failure was induced by rapidly pacing the left ventricle at 200 beats/min. Vacuum assistance of −20 mmHg was applied, the BioVAD settings were adjusted to maintain blood flow of approximately 50 ml/kg/min, and the animal was supported for 4 hours. No adjustments were made in the BioVAD settings, and hemodynamic parameters were recorded every 30 minutes.
Statistical methods
Results are expressed as means +/− standard deviation. For comparisons of parametric variables, Students t-test was applied, paired or unpaired as appropriate, using Excel (Microsoft Inc, Redmond, WA). P <0.05 was considered significant.
Results
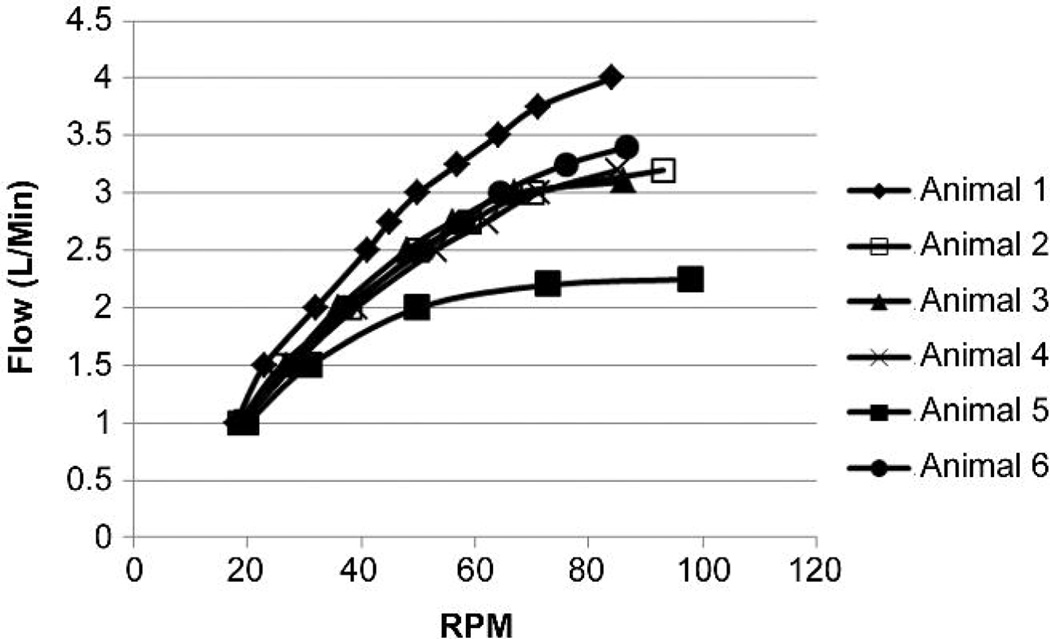
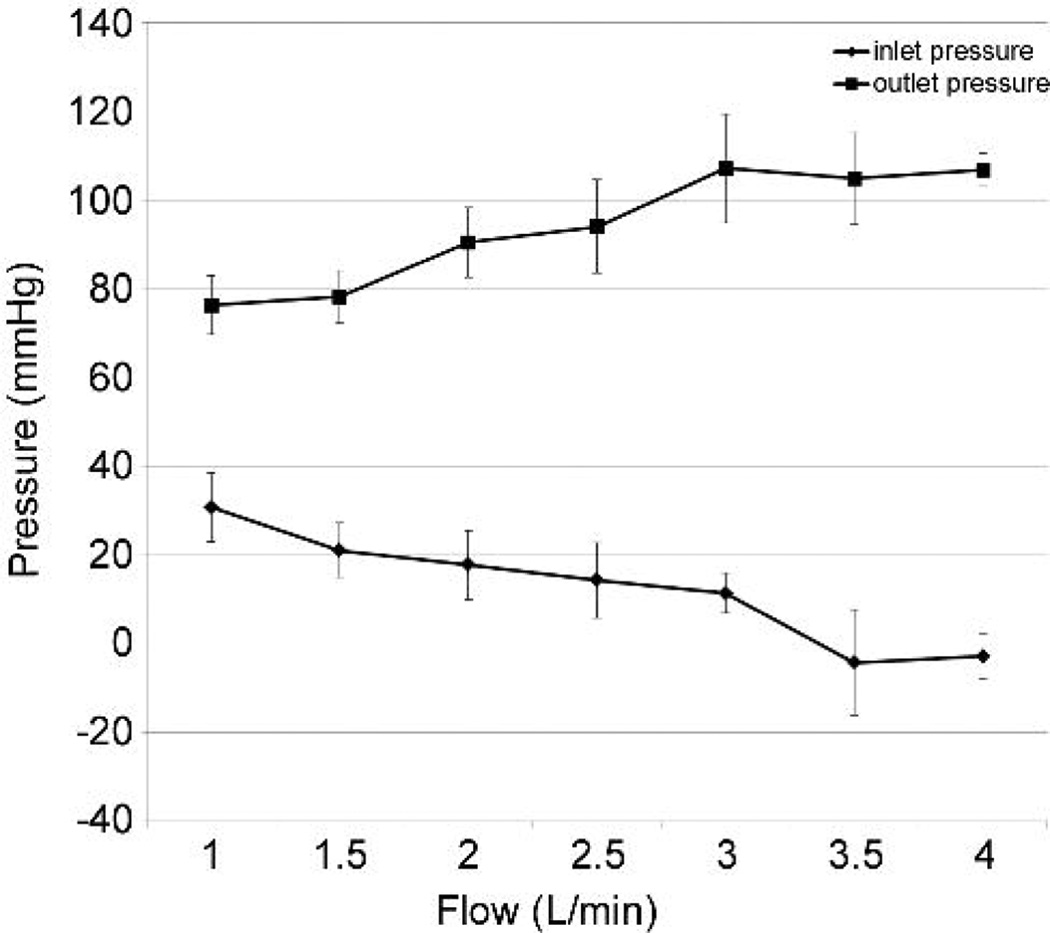
Pump flow increased with BioVAD rotational speed until plateau was observed (Figure 3). RPM speed at plateau varied from 84 to 98, with a mean of 88.8 +/− 5.5. The addition of vacuum further augmented flow from 3.19 +/− 0.56 L/min to 3.71 +/− 0.53 L/min. Peak flow was achieved with an average left atrial pressure of 9.5 +/− 1.4 mmHg, a mean arterial pressure of 65.8 +/− 23.0 mmHg, a pump inlet pressure of 7.7 +/− 6.9 mmHg and a pump outlet pressure of 106.3 +/− 33.7mmHg. Pump inlet pressure only became negative with vacuum assistance, and to a minimal degree (Figure 4). Pump outlet pressure was less than 120 mmHg, and reached plateau well before maximum flow was achieved (Figure 4).
Figure 3.
A: Pump flow versus rotational speed.
Figure 4.
Inlet and outlet pressure versus pump flow.
During one liter of rapid volume infusion, left atrial pressure rose from 10.1 +/− 1.6 to 20.0 +/− 4.4 mmHg. Without changes in BioVAD settings, pump output increased by an average of 17.5% from 2.96 +/− 0.58 to 3.48 +/− 0.70 L/min (p=0.007). During volume contraction after 500 ml phlebotomy, left atrial pressure fell from 13.5 +/− 2.6 to 9.5 +/− 1.8 mmHg. Flow generated by the BioVAD decreased by an average of 7.8% from 3.36 +/− 0.63 to 3.10 +/− 0.56 (p=0.005).
After creation of systemic hypertension by injection of phenylephrine 200–400 mcg, mean arterial pressure increased from 70.7 +/− 11.2 to 168.0 +/− 9.0 mmHg. Pump flow did not significantly change, from 3.31 +/− 0.59 L/min to 3.51 +/− 0.79 L/min, p=0.22. After baseline hemodynamic parameters were reestablished, 20 mm Hg vacuum was applied to achieve maximum flow. The inlet and outlet lines were alternately abruptly clamped, and the circuit was observed and filmed. Inlet suction never exceeded negative 150 mmHg and there was no evidence of cavitation (Table 1). Peak outlet pressure was approximately 600 mmHg, with no circuit disruption or BioVAD malfunction.
Table 1.
| Clamp position/Variable | Pressure (mmHg) |
+/− SD | Cavitation/Malfunction |
|---|---|---|---|
| Inlet | −88 (Mean) | 29 | No |
| Inlet | −127 (Peak) | 18 | No |
| Outlet | 395 (Mean) | 82 | No |
| Outlet | 610 (Peak) | 104 | No |
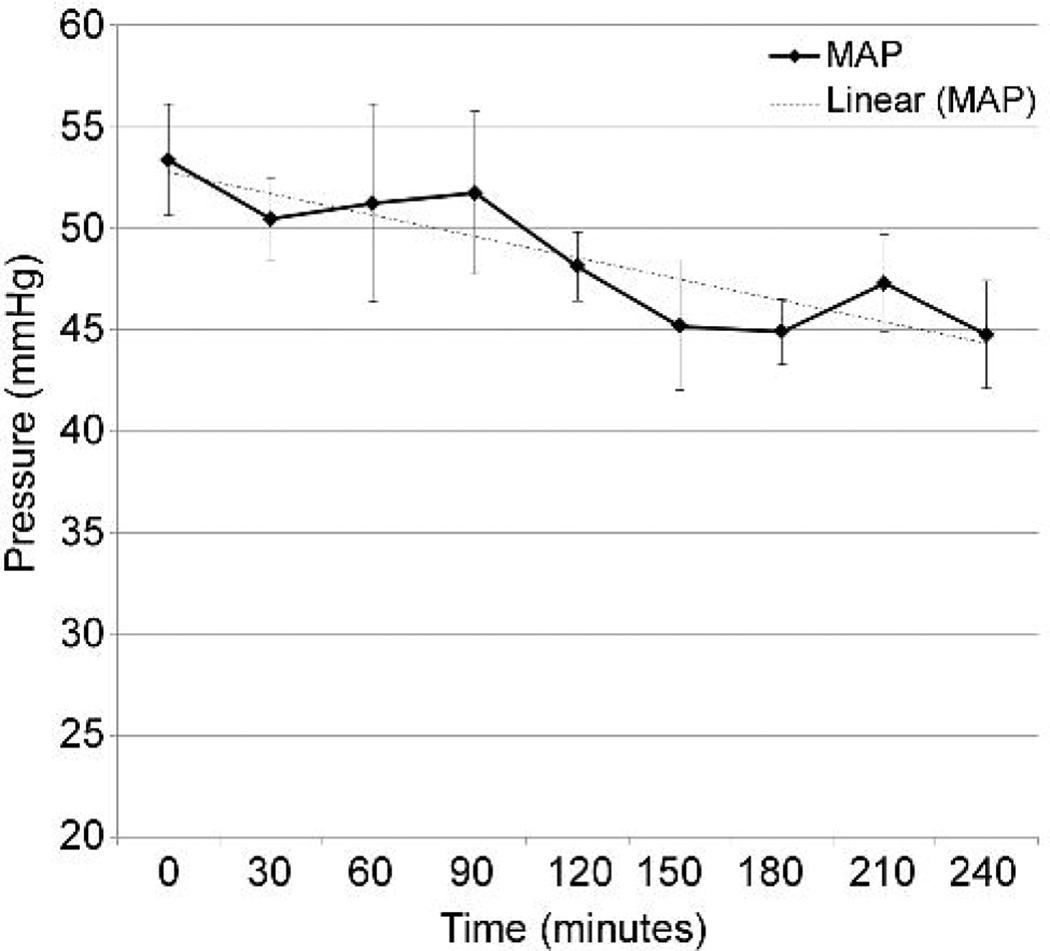
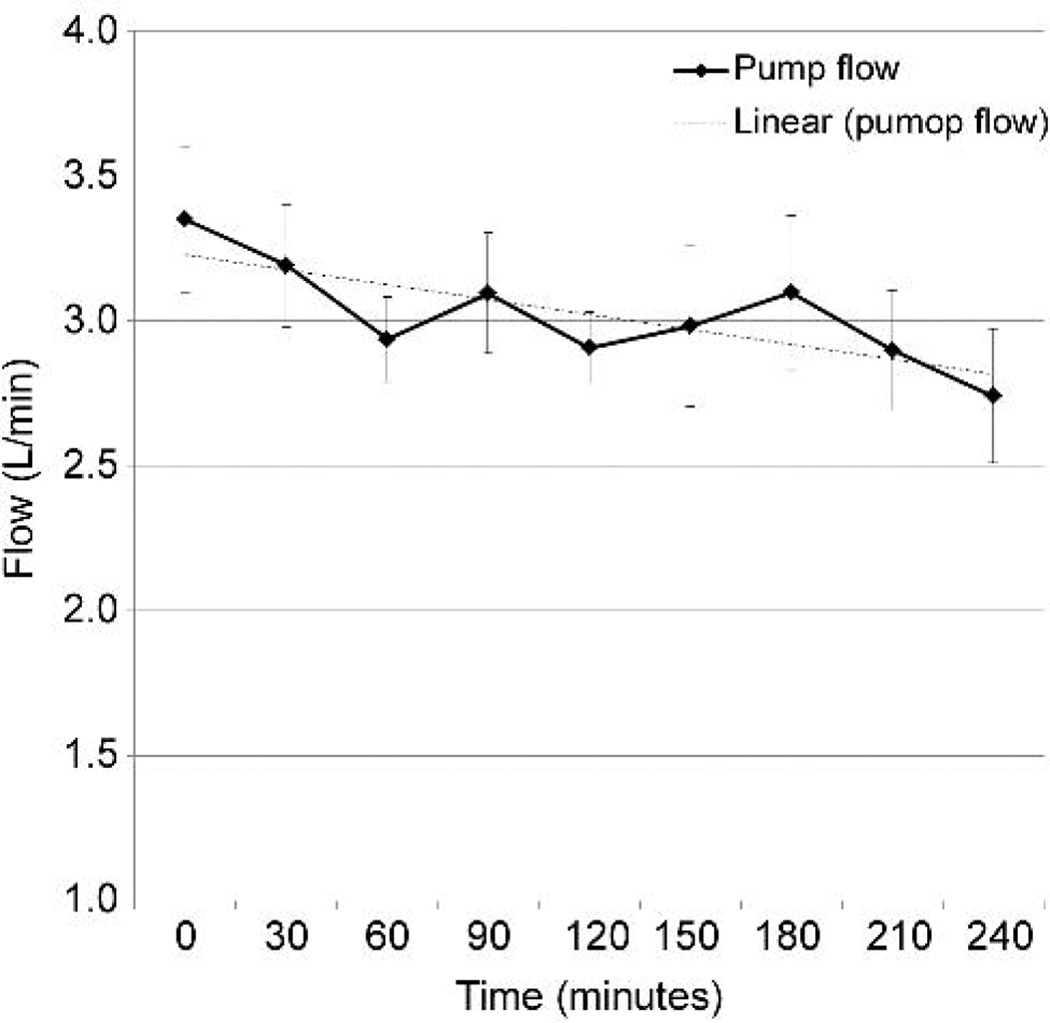
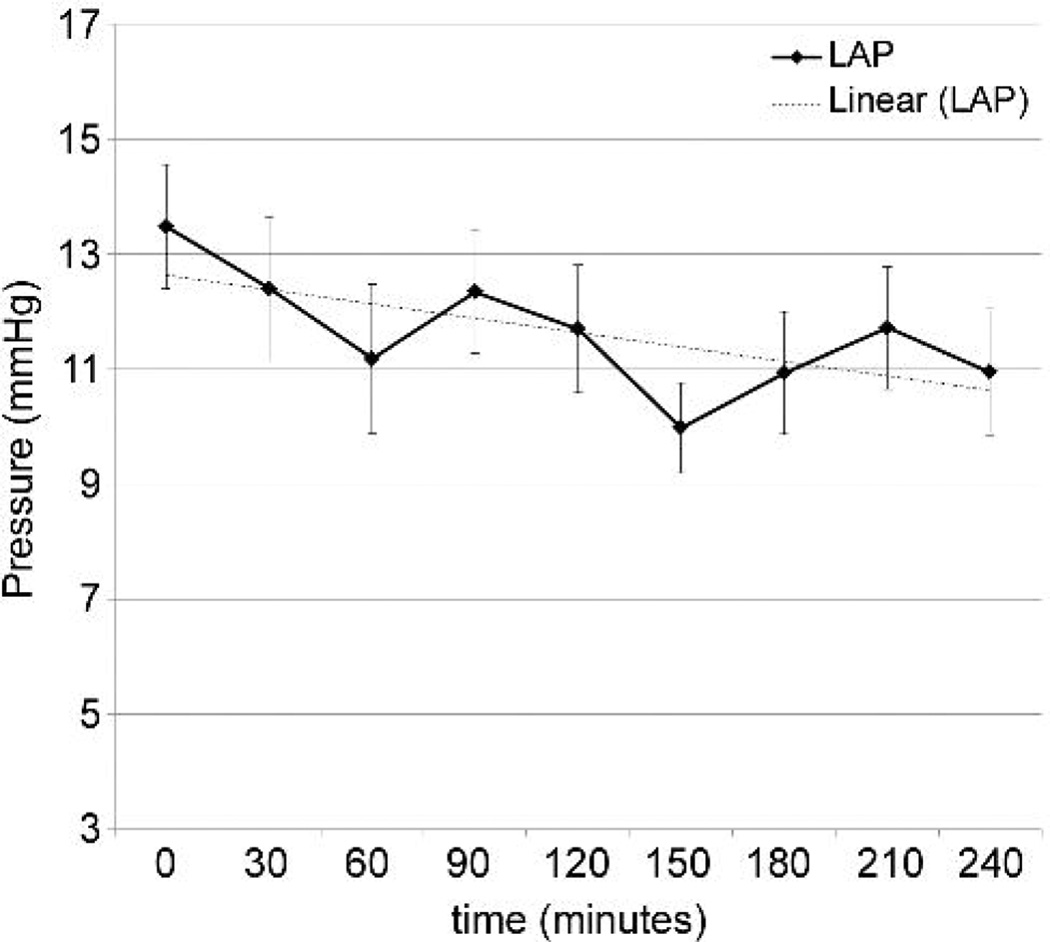
Acute heart failure was induced by rapid ventricular pacing at 200 beats/minute using epicardial right ventricular leads. Arterial line tracing demonstrated minimal residual left ventricular ejection compared to baseline. Pump RPM and vacuum were retained at pre-established settings and not adjusted. Mean arterial blood pressure was maintained near 50 mmHg, with some decline over time (Figure 5A). Average pump flow at initiation of heart failure was 3.35 L/min, and declined over the course of the support (Figure 5B), associated with a decline in left atrial pressure (Figure 5C).
Figure 5.
Supported heart failure model. A: Mean arterial pressure. B: BioVAD flow. C: Left atrial pressure.
Comment
Implantable VADs are increasingly utilized for patients with end stage heart failure6–8. The devices are highly engineered for excellent biocompatibility, durability, and reliability in an ambulatory setting, capable of supporting patients continuously for years. These devices cost approximately $100,000, and the implant techniques and care protocols are complex. As such, they are typically only employed at highly specialized centers and rarely applied to patients in profound cardiogenic shock9. In the most severe cases of shock, patients require high dose vasoconstrictors or even cardiopulmonary resuscitation and may have suffered devastating neurologic injury, intestinal infarction, shock liver and other forms of potentially irreversible end organ damage. Temporary extracorporeal mechanical circulatory support can stabilize hemodynamics, restore end organ function, and allow time to assess either the likelihood of cardiac recovery or to determine the patient’s suitability for long-term cardiac replacement with an implantable VAD or heart transplantation10. This paradigm, referred to as “bridge to decision” employs a variety of available devices in a number of applications, including peripheral and central ECMO11,12, percutaneous trans-septal left ventricular assistance13, and surgically implanted temporary VADs2,4.
Different mechanical blood pumps can be used for these applications, but regardless of the mode and location of cannulation or the presence of an oxygenator, the same basic principles of an ideal pump should be applicable. The pump must be safe, easy to manage in potentially dynamic physiologic conditions, and applicable to the spectrum of approaches used in temporary circulatory assistance. Low thrombogenicity and biocompatibility are also essential to reduce device related complications.
The BioVAD studied in this report was designed to satisfy these general principles. Flow rates achieved were well within the expected performance range of the pump, at speeds of 50–100 RPM. Inter-animal variability in flow could be related to cannula position, with possible partial obstruction by the interventricular septum or the posterior wall. Future studies will include careful necropsy to identify and document possible explanations for these variances. Inlet suction was avoided during normal operation as well as during simulated line occlusion, a situation which otherwise could be associated with cavitation, hemolysis and potentially other unwanted adverse events. The pump intrinsically demonstrated a Starling response to extreme fluctuations in intravascular volume. The BioVAD was also afterload insensitive, with maintained support despite abrupt changes in afterload.
The BioVAD represents the evolution of a pump originally developed to improve safety during cardiopulmonary bypass (CPB)15. It’s predecessor, the M-Pump (MC3 Inc, Ann Arbor, MI), is also capable of functioning as a short term VAD or ECMO pump16, but it has a large physical footprint and requires gravity siphon for drainage. While these features may be irrelevant for CPB, they are unsuitable for prolonged use in the ICU setting. The BioVAD was miniaturized to reduce its profile and improve portability and ease of care. In addition, the enclosure was sealed to allow vacuum assisted drainage, eliminating the requirement for gravity dependent siphon, thus shortening the circuit and reducing prime, surface area, and blood transit time.
Previous in vitro testing demonstrated that the BioVAD retained its Starling response to volume loading, although not to the same degree as the M-Pump5. Alterations to the BioVAD pump chamber volume or pump-head diameter can improve the Starling response, although the 17% increase in pump output seen with volume loading during this in vivo study may be sufficient responsiveness. Some negative pressure was observed with inlet clamping from fluid leakage into the pump chamber. The suction generated was reasonably low and unlikely to impart significant clinical implications. Additional refinements to the pump chamber fabrication processes are expected to further improve collapsibility and performance.
In this model, we studied apical cannulation for drainage, as is typically used in an LVAD. The apical approach was chosen because of the relatively small size of the left atrium in normal healthy sheep and concerns of cannula obstruction from atrial wall collapse. Left ventricular drainage affects pump filling because of the pumping action of the heart and the positive systolic pressure generated. We must assess how the BioVAD will perform in the absence of this augmented drainage. We plan to perform short term studies using direct right atrial drainage (RVAD configuration) and percutaneous right atrial drainage with the addition of an oxygenator for ECMO support. The latter approach will also demonstrate how the pump responds with the additional afterload of the oxygenator and the blood flow resistance of peripheral cannulae. Additional vacuum may be required to overcome the pressure drop of peripheral cannulae and maintain inflow to the pump chamber. We anticipate that suitable flow rates and volume responsiveness can be met in these circumstances.
Future studies will include LVAD, RVAD, and ECMO configurations for 24 hours to assess for hemolysis, platelet consumption, inflammatory response, early thrombus, and evidence of end organ dysfunction or complications. Another unique feature of the BioVAD design is that the blood contacting surface can be made without seams and entirely coated with a variety of biocompatible surfaces from cannula to cannula. We plan to test a variety of surface coatings and assess for thrombogenicity over five day recovery experiments, completed both with and without heparin infusions. The BioVAD will also be compared to currently used centrifugal and pneumatic pulsatile pumps.
Conclusions
The BioVAD prototype is a novel blood pump with features which may afford superiority for short term circulatory support. Unlike centrifugal pumps, negative inlet pressures are avoided, thus eliminating the risks of cavitation and hemolysis. The pump is also afterload insensitive and the blood contacting surfaces are valveless and uniform. The initial BioVAD prototype produced flow exceeding 60mL/kg/min in an acute animal model and maintained hemodynamic support for 4 hours.
Acknowledgments
This study was supported by NIH 42HL096168.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Haft JW et al. Short- and long-term survival of patients transferred to a tertiary care center on temporary extracorporeal circulatory support. Ann Thorac Surg. 2009;88(3):711–717. doi: 10.1016/j.athoracsur.2009.04.007. discussion 717–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson M, et al. Use of the AB5000 ventricular assist device in cardiogenic shock after acute myocardial infarction. Ann Thorac Surg. 90(3):706–712. doi: 10.1016/j.athoracsur.2010.03.066. [DOI] [PubMed] [Google Scholar]
- 3.Ziemba EA, John R. Mechanical circulatory support for bridge to decision: which device and when to decide. J Card Surg. 25(4):425–433. doi: 10.1111/j.1540-8191.2010.01038.x. [DOI] [PubMed] [Google Scholar]
- 4.Hemmila MR, et al. Extracorporeal life support for severe acute respiratory distress syndrome in adults. Ann Surg. 2004;240(4):595–605. doi: 10.1097/01.sla.0000141159.90676.2d. discussion 605–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spurlock DJ, et al. Bench-Top Evaluation of a Novel Extracorporeal Device for use in Acute Cardiogenic Shock. ASAIO Journal. (In Press) [Google Scholar]
- 6.Pagani FD, et al. Extended mechanical circulatory support with a continuous-flow rotary left ventricular assist device. J Am Coll Cardiol. 2009;54(4):312–321. doi: 10.1016/j.jacc.2009.03.055. [DOI] [PubMed] [Google Scholar]
- 7.Rose EA, et al. Long-term use of a left ventricular assist device for end-stage heart failure. N Engl J Med. 2001;345(20):1435–1443. doi: 10.1056/NEJMoa012175. [DOI] [PubMed] [Google Scholar]
- 8.Slaughter MS, et al. Advanced heart failure treated with continuous-flow left ventricular assist device. N Engl J Med. 2009;361(23):2241–2251. doi: 10.1056/NEJMoa0909938. [DOI] [PubMed] [Google Scholar]
- 9.Kirklin JK, et al. Third INTERMACS Annual Report: the evolution of destination therapy in the United States. J Heart Lung Transplant. 30(2):115–123. doi: 10.1016/j.healun.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 10.Lewis EF, et al. Frequency and impact of delayed decisions regarding heart transplantation on long-term outcomes in patients with advanced heart failure. J Am Coll Cardiol. 2004;43(5):794–802. doi: 10.1016/j.jacc.2003.10.035. [DOI] [PubMed] [Google Scholar]
- 11.Chen YS, et al. Conversion of extracorporeal membrane oxygenation to non-pulsatile left ventricular assist device. Is it out-of-date for non-pulsatile LVAD? J Cardiovasc Surg (Torino) 2001;42(4):457–463. [PubMed] [Google Scholar]
- 12.Smedira NG, Blackstone EH. Postcardiotomy mechanical support: risk factors and outcomes. Ann Thorac Surg. 2001;71(3 Suppl):S560–S566. doi: 10.1016/s0003-4975(00)02626-6. discussion S82–5. [DOI] [PubMed] [Google Scholar]
- 13.Kar B, et al. Clinical experience with the TandemHeart percutaneous ventricular assist device. Tex Heart Inst J. 2006;33(2):111–115. [PMC free article] [PubMed] [Google Scholar]
- 14.Caccamo M, Eckman P, John R. Current state of ventricular assist devices. Curr Heart Fail Rep. 8(2):91–98. doi: 10.1007/s11897-011-0050-z. [DOI] [PubMed] [Google Scholar]
- 15.Montoya JP, Merz SI, Bartlett RH. Bartlett, Laboratory experience with a novel, non-occlusive, pressure-regulated peristaltic blood pump. ASAIO J. 1992;38(3):M406–M411. doi: 10.1097/00002480-199207000-00065. [DOI] [PubMed] [Google Scholar]
- 16.Montoya JP, Merz SI, Bartlett RH. Bartlett, Significant safety advantages gained with an improved pressure-regulated blood pump. J Extra Corpor Technol. 1996;28(2):71–78. [PubMed] [Google Scholar]