Abstract
Background and aims
Micro-morphological characteristics can influence fungal infectivity. We sought links between micro-morphology and resistance to powdery mildew in mulberry with the intention of assisting selection of disease-resistant lines.
Methodology
Over 3 years and under field conditions, we evaluated 30 lines of mulberry with contrasting susceptibilities to powdery mildew (15 resistant and 15 susceptible). Disease severity was related statistically to stomatal area, stomatal density, stomatal index, upper and lower cuticular thicknesses, leaf thickness and trichome density.
Principal results
Differences between lines were significant (P <0.05) for all characters studied. Variation between the resistant and susceptible groups was statistically highly significant (P <0.01) for stomatal index, stomatal area and trichome density. The powdery mildew-resistant group was distinguished by 17.4 % lower stomatal density, 12.5 % smaller stomatal index per unit leaf area, 20.0 % greater trichome density and 18.0 % higher stomatal area compared with the susceptible group. Trichome density was negatively correlated with disease severity index and with the accumulative area under disease progression curves. Stomatal density was positively correlated with both measures of disease severity. Although stomatal area was negatively related to disease severity index (r = −0.28; P <0.05), the correlation was weak. There was no statistically significant relationship between stomatal area and the accumulative area under disease progression curves. The germplasm was partitioned into seven sub-groups based on hierarchical cluster analysis derived from pooled disease severity index scores and three highly significant micro-morphological characters. Eighty per cent of the resistant germplasm accumulated in three cluster components (A1, A2 and B2) characterized by high trichome densities and a high stomatal density and stomatal index.
Conclusions
Resistance to powdery mildew in mulberry is associated with trichome and stomatal features rather than leaf and epidermal thicknesses. Trichome density, stomatal density and stomatal index are shown to be promising markers for screening powdery mildew resistance in breeding programmes.
Introduction
Mulberry (Morus spp.) is cultivated widely in geographical areas that include temperate and subtropical regions of the northern hemisphere and tropical parts of the southern hemisphere. Its main use is as the primary food source for the silkworm Bombyx mori (Sharma et al. 2000). Powdery mildew, an obligate biotrophic ascomycete fungus [Phyllactinia corylea (syn. P. guttata syn. P. moricola); Itoi et al. 1982; Brown 2002; Takamatsu et al. 2008], is one of the major diseases of mulberry throughout the world. The disease is characterized by white dust-like mycelia that develop over abaxial leaf surfaces. Heavily infected tissues develop chlorosis and senesce prematurely (Gupta 2001). The resulting foliage loss, typically 20 %, reduces substantially the yield of silkworm cocoons (Manimegalai and Chandramohan 2007). Disease control through inherent plant resistance is desirable since it would reduce dependence on costly fungicides that can damage both the environment and the silkworms themselves (Govindaiah and Gupta 2005). Therefore, from both economic and environmental points of view, the development of resistant cultivars is highly attractive.
In a recent screening programme of 147 individuals of various Morus species, 6 % of the germplasm showed powdery mildew resistance under both natural and artificial epiphytotics (Chattopadhyay et al. 2010). However, mulberry cultivar development is made slow by high heterozygosity and the time required for trees to mature to flowering. The process may be further delayed by reliance on field observations of disease levels, which can be confounded by year-on-year environmental variations. A faster approach would be to seek dependable markers for disease resistance. Accordingly, we have sought readily scored defence-related traits in leaf micro-morphology. This approach may also benefit long-term improvement in other crops (Kolkman and Kelly 2002; Gabler et al. 2003).
Plant defence against pathogen attack is complex, with many local and systemic aspects (Felle et al. 2004). The internal anatomy and surface features of the leaves often determine plant resistance to biotrophic pathogen infection (Smith et al. 1996). Among such characters, aspects of stomata, cuticle and trichome morphology can influence disease resistance (Niks and Rubiales 2002). Unlike other powdery mildew pathogens, the genus Phyllactinia is partly (hemi) endoparasitic (Glawe 2008). The pathogen indirectly penetrates the mesophyll via stomata to form haustoria (Takamatsu et al. 2008). Therefore, stomata play an important role for Phyllactinia infection of compatible hosts (Braun et al. 2002). However, stomata-penetrating pathogens need appropriate cues to locate stomatal pores after they adhere to the leaf surface (O'Connell and Panstruga 2006). Trichomes can act as physical barriers to infection (Martin and Glover 2007) and, in Uromyces, this can retard germination on the surface of bean leaves by trapping the spores (Mmbaga et al. 1994), thereby reducing the probability of germ tubes reaching the penetration site (Wynn 1976). A high frequency of trichomes can also prevent mycelial penetration and infection of other biotrophic fungi (Shaik 1985). Leaf and cuticular or epidermal thicknesses have also been associated with powdery mildew resistance (Commenil et al. 1997). Indeed, biotrophy by powdery mildew is initially dependent upon the morphology of the epidermal surface and the adhesion capacity of pathogen spores to the cuticle/cell wall (Vanacker et al. 2000; Zeyen et al. 2002). In mulberry, germplasm variation in these micro-morphological attributes has been reported (Biasiolo et al. 2004; Banerjee et al. 2006). However, relationships between these characters and resistance to powdery mildew have not been explored before in mulberry.
Our objectives were to (i) evaluate powdery mildew resistance of 30 lines of mulberry previously regarded as resistant or susceptible, (ii) quantify several micro-morphological characters of leaves from resistant and susceptible plants and (iii) determine the degree of association between foliar micro-morphological characters and powdery mildew resistance.
Materials and methods
Carefully chosen lines in the form of rooted saplings of Morus spp. were evaluated in the experimental garden of Central Sericultural Research and Training Institute in Berhampore, India (19 m a.s.l.; 24°6′N and 88°15′E). Saplings from 30 accessions (15 putatively resistant and 15 putatively susceptible) were planted in an augmented randomized block design with 0.6 m × 0.6 m spacing. Three local susceptible accessions (‘Bishnupur-4’, ‘Matigara Black’ and ‘C-2016’) were placed every six rows in a completely randomized manner. A spreader row of ‘Kolitha-3’ was placed around all sides to encourage a uniform distribution of mildew conidia in the experimental field. The number of test plants was 14 per accession over two sub-plots separated by a spreader row. Three rounds of powdery mildew screening were conducted during October–November (peak season of disease incidence in West Bengal) spanning 2006–2008. The timing coincided with the ‘Agrahyani' commercial silkworm rearing schedule of West Bengal.
Disease assessments
Ten plants of each line were scored visually for percentage of leaf area covered by powdery mildew on a 0 (resistant) to 10 (susceptible) scale four times between 32 and 62 days after ground-level pruning in each year. For analysis, disease scores were converted using the modified logarithmic scale of Horsfall–Barrett (Horsfall and Cowling 1978). The scale was 0 = 0 %, 1 = 0–3 %, 2 = +3–6 %, 3 = +6–12 %, 4 = +12–25 %, 5 = +25–50 %, 6 = +50–75 %, 7 = +75–88 %, 8 = +88–94 %, 9 = +94–97 % and 10 = +97–100 %. Disease severity index (DSI) was calculated according to Kim et al. (2000) using the following formula:
Powdery mildew development over time was assessed as the area under a disease progression curve (AUDPC) according to the formula of Campbell and Madden (1990) as follows:
where n is the number of evaluation times, xi is the disease intensity at evaluation time i and ti+1 is the time between two disease scores.
Foliar anatomical parameters
Foliar parameters were measured on three consecutive occasions. Sixty-two days after pruning to ground level, the fourth and fifth leaves from the tip of shoots from each accession were placed in plastic bags with a wetted filter and removed to the laboratory for stomatal and anatomical studies. Mulberry is hypostomatous, and stomatal features were assessed using impressions of abaxial leaf surfaces taken at the point of maximum leaf width near the mid-vein using colourless nail polish and adhesive transparent cellophane tape. All impressions were fixed on glass slides and examined under a light microscope at ×100 magnification. Stomata were counted and stomatal density (SD) was calculated as the number of stomata per unit leaf area (mm2). The stomatal index (SI) was calculated according to Ferris and Taylor (1994) using the formula SI (%) = [(stomata)/(total cells + stomata)] × 100. For stomatal area (SA), 20 randomly selected stomata were measured microscopically using an ocular micrometer (Mishra 1997). Stomatal length was defined as the length of the long axis of the area bounded by the outer stomatal ledges; width was the length of the short axis between the edges of the outer ledges. Stomatal area (µm) was calculated for each stoma using the equation SA = πab, where a and b are 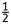 length and
length and 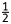 width, respectively. We assumed that the opening between outer ledges was a perfect ellipse (Wise et al. 2000). Trichomes were counted from five microscopic fields per leaf sample along the mid-vein side using a Diaplan stereo-dissecting microscope (Lietz M-8) and every half field was converted to number of trichomes per square millimetre (Valverde et al. 2001).
width, respectively. We assumed that the opening between outer ledges was a perfect ellipse (Wise et al. 2000). Trichomes were counted from five microscopic fields per leaf sample along the mid-vein side using a Diaplan stereo-dissecting microscope (Lietz M-8) and every half field was converted to number of trichomes per square millimetre (Valverde et al. 2001).
Anatomical determinations of abaxial and adaxial cuticle thicknesses (CTb and CTd) and leaf thickness (LT) were made on thick transverse cross-sections (ca. 70 µm) at the point of maximum leaf width. Leaf samples were fixed in formalin–acetic acid–ethanol (1:1:18) for 18 h, dehydrated by passing through ethanol grades and solidified into paraffin (melting point 56 °C) blocks according to Vijayan et al. (2008). The sections were made by a rotary microtome.
Data analyses
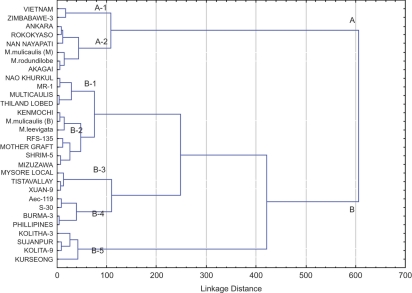
Analysis of variance was performed using Microsoft Excel version 8.0. When F values were significant (P <0.05), Fisher's least significant differences were calculated. Heterogeneity in the variances was observed in the data from disease screening methods. Accordingly, arcsine-square root transformations were applied before analysis. Student's t-tests for paired two-sample means were used to compare resistant and susceptible germplasm groups. Pearson's correlation coefficients were calculated to compare disease ratings with leaf micro-morphological characters for mean values of each germplasm line (Gomez and Gomez 1984). Further, DSI and AUDPC along with promising micro-morphological values were clustered using ‘Statistica’ version 8.0 software (Statsoft Inc., Tulsa, OK, USA). The Euclidean distance based on the complete amalgamation rule was used to construct a dendrogram using hierarchical clustering. A linkage distance of 40 was arbitrarily chosen to separate the germplasm into seven clusters in the dendrogram (Berdahl et al. 1999).
Results
Significant germplasm variance was observed for DSI, disease progression curves (AUDPC) and all micro-morphological parameters measured (Table 1). Germplasm variances for SI, SD and trichome density (TD) were influenced by year of data collection (P ≤0.01), but such variation was non-significant for DSI, AUDPC, SA, CTd, CTb and LT (P >0.05). Germplasm×year effects were also non-significant for all tested parameters (P >0.05). Moreover, variance component estimates for germplasm were greater than estimates for the year and germplasm×year interaction in all parameters except LT.
Table 1.
Analysis of variance of micro-morphological leaf characteristics of 30 lines of mulberry and their responsiveness to powdery mildew. Evaluations were made over 3 years (2006–2008) in a field environment at Berhampore, West Bengal, India.
| Source | Mean squares |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| df | DSIa | AUDPCb | SIc | SZd | SDe | CTbf | CTdg | LTh | TDi | |
| Germplasm | 29 | 1529.3** | 19878.9 | 257.2** | 9933.5** | 23717.9** | 1.748* | 0.418* | 4322.1** | 194.11** |
| Year | 2 | 0.695ns | 910.9ns | 29.0 | 1215.5ns | 1125.5ns | 0.0161ns | 0.012ns | 8636.5ns | 96.86** |
| Year×germplasm | 58 | 0.915ns | 178.4ns | 1.67ns | 230.5ns | 65.9ns | 0.0211ns | 0.014ns | 3840.9ns | 2.54ns |
** and * indicate significance at P< 0.01 and P < 0.05, respectively.
aDisease severity index; baccumulative area under disease progression; cstomatal index; dstomatal density (no. mm−2); eindividual stomatal size (µm2); fabxial and gadaxial cuticle thicknesses (µm); hleaf thickness (µm); itrichome density (no. mm−2).
Mean powdery mildew scores ranged from 1.6 to 43.8 (variation 27.3-fold) for DSI and from 31.1 to 520.2 (16.7-fold) for AUDPC (Table 2). The most mildew-susceptible germplasm ‘Philippines’ showed 40–51 % of its leaf area to be affected by the last evaluation date. No line was completely immune to powdery mildew. Reactions to powdery mildew of selected lines (resistant and susceptible) were stable across years (data not shown). Irrespective of resistance and susceptible disease reactions, CTb was significantly higher than CTd for all germplasm (Table 2). Significant differences in SA, epidermal thicknesses and LT were observed among germplasm. Even though mean SA and LT of powdery mildew-resistant germplasm were, respectively, 18 and 6.9 % less than those of the susceptible group, the difference between the two groups was statistically non-significant for these two parameters and for epidermal thicknesses. Significant differences in SD, SI and TD were also found among the mulberry germplasm. Unlike for SA, CTb, CTd and LT, the differences between resistant and susceptible germplasm groups (measured by Student's t-test) were also significant for these three features. The mean differences of SD, SI and TD in powdery mildew-resistant and mildew-susceptible reaction groups were 117.2, 3.3 and 4.1, respectively. The resistant germplasm had 20 % more TD with 12.5 and 17.4 % less SI and SD than the susceptible germplasm group.
Table 2.
Disease severity of micro-morphological leaf characteristics of 30 lines of mulberry with contrasting susceptibility to powdery mildew. Mean values are shown for DSI, area under disease progression curve, SA, cuticular and leaf thicknesses obtained under field conditions at Berhampore, West Bengal, India, over 3 years (2006–2008).
| Accession | Name | Species | Origin | DSIa | AUDPCb | SIc | SDd | SAe | CTbf | CTbg | LTh | TDi |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EC-493900 | Vietnam-2 | M. multicaulis | Vietnam | 1.6 | 31.8 | 20.3 | 426.6 | 171.7 | 1.9 | 0.9 | 112.6 | 20.4 |
| EC-493982 | Ankara | Morus spp. | Turkey | 3.9 | 61.4 | 18.0 | 479.3 | 134.5 | 2.2 | 1.2 | 123.2 | 21.5 |
| – | M.multicaulis (M) | M. multicaulis | Indonesia | 6.9 | 38.9 | 23.8 | 524.4 | 171.7 | 2.4 | 1.1 | 126.0 | 23.4 |
| IC-313791 | Nao-khurkul | Morus spp. | India | 7.3 | 45.5 | 26.6 | 664.1 | 224.6 | 3.4 | 1.5 | 143.7 | 20.0 |
| EC-493796 | Kenmochi | Japan | 9.1 | 66.5 | 22.5 | 606.7 | 171.2 | 2.5 | 0.8 | 142.7 | 22.0 | |
| – | Multicaulis | M. multicaulis | Indonesia | 9.5 | 85.8 | 25.3 | 645.1 | 171.2 | 1.7 | 0.8 | 117.3 | 21.6 |
| EC-493973 | Rotundiloba | M. rotundiloba | Myanmar | 8.4 | 55.4 | 21.6 | 506.6 | 173.2 | 2.7 | 1.1 | 136.7 | 21.5 |
| EC-493895 | M. multicaulis (B) | M. multicaulis | Indonesia | 11.6 | 101.1 | 24.3 | 610.7 | 269.2 | 2.5 | 1.0 | 121.5 | 21.4 |
| IC-313861 | Non-nayapati | Morus spp. | India | 12.0 | 94.5 | 22.1 | 489.5 | 151.1 | 2.4 | 1.2 | 127.2 | 17.1 |
| EC-493819 | Rokokyaso | M. latifolia | Japan | 11.5 | 112.4 | 20.4 | 479.3 | 212.8 | 2.1 | 0.8 | 123.9 | 19.2 |
| EC-49352 | Thailand lobed | M. alba | Thailand | 11.6 | 72.7 | 25.8 | 646.8 | 223.8 | 2.4 | 1.3 | 134.5 | 23.1 |
| IC-313976 | MR-1 | M. sinensis | India | 13.0 | 118.2 | 25.4 | 668.7 | 151.2 | 1.7 | 1.0 | 118.0 | 20.3 |
| IC-314137 | Laevigata | M. laevigata | India | 12.9 | 90.4 | 24.4 | 587.0 | 146.3 | 1.8 | 0.9 | 120.6 | 20.6 |
| EC-493856 | Zimbabwe-3 | Morus spp. | Zimbabwe | 13.3 | 125.4 | 18.4 | 423.5 | 130.7 | 2.6 | 1.2 | 135.5 | 13.8 |
| EC-493842 | Akagai | M. bombycis | Japan | 13.7 | 102.1 | 25.8 | 558.2 | 200.5 | 2.3 | 1.0 | 125.2 | 21.9 |
| Mean | 9.8 | 80.1 | 23.0 | 554.4 | 179.8 | 2.3 | 1.1 | 127.2 | 20.5 | |||
| IC-313694 | RFS-135 | M. indica | India | 29.8 | 269.7 | 26.6 | 618.9 | 132.3 | 1.6 | 0.8 | 120.8 | 13.2 |
| EC-493798 | Shrim-5 | M. alba | Bangladesh | 36.8 | 390.5 | 20.8 | 618.2 | 165.8 | 2.2 | 1.0 | 120.6 | 11.6 |
| IC-313733 | Kolitha-3 | M. indica | India | 30.4 | 322.2 | 34.8 | 947.4 | 163.4 | 1.5 | 0.6 | 93.8 | 12.9 |
| IC-313697 | Mysore local | M. indica | India | 30.0 | 312.0 | 31.3 | 839.8 | 145.0 | 2.2 | 0.9 | 132.9 | 11.5 |
| IC-313832 | Kurseong | M. indica | India | 30.8 | 279.3 | 33.8 | 979.3 | 153.6 | 2.3 | 1.0 | 135.6 | 12.4 |
| IC-313667 | Mother graft | Morus spp. | India | 30.3 | 245.6 | 23.4 | 629.9 | 147.0 | 2.4 | 0.9 | 132.6 | 12.4 |
| EC-493791 | Mizuzawa | M. bombysis | Japan | 32.2 | 273.5 | 24.8 | 618.7 | 156.9 | 1.5 | 0.7 | 106.6 | 8.7 |
| IC-313687 | Acc 119 | Morus spp. | India | 30.9 | 242.9 | 27.5 | 732.1 | 126.2 | 2.0 | 1.0 | 112.7 | 15.0 |
| IC-313821 | Tista valley | M. alba | India | 32.5 | 333.0 | 32.3 | 826.0 | 155.2 | 2.1 | 1.0 | 107.2 | 14.0 |
| IC-313692 | S-30 | M. indica | India | 31.9 | 181.1 | 31.1 | 734.2 | 163.5 | 1.9 | 1.0 | 104.1 | 11.1 |
| EC-493901 | Xuan-9 | Morus spp. | China | 37.4 | 448.0 | 30.8 | 931.2 | 159.4 | 2.7 | 1.3 | 114.1 | 13.0 |
| IC-313872 | Sujanpur local | M. alba | India | 36.3 | 378.3 | 37.7 | 947.2 | 121.4 | 2.5 | 1.3 | 121.2 | 12.2 |
| IC-313820 | Kolitha-9 | M. indica | India | 42.6 | 438.9 | 37.0 | 980.9 | 143.4 | 2.7 | 1.2 | 130.6 | 10.6 |
| EC-493777 | Burma-8 | M. indica | Myanmar | 40.9 | 445.3 | 26.5 | 762.2 | 127.2 | 2.3 | 1.0 | 112.5 | 12.3 |
| EC-493768 | Philippines | M. indica | Philippines | 43.8 | 520.2 | 22.8 | 765.3 | 149.3 | 3.1 | 1.3 | 134.0 | 13.1 |
| Mean | 34.5 | 338.7 | 29.5 | 788.7 | 147.3 | 2.2 | 1.0 | 118.6 | 12.3 | |||
| Grand mean | 22.1 | 209.4 | 26.3 | 671.6 | 163.6 | 2.3 | 1.0 | 122.9 | 16.4 | |||
| LSD(0.05) | 1.74 | 28.9 | 2.59 | 70.7 | 44.4 | 0.47 | 0.33 | 4.0 | 4.07 | |||
| t-value res. vs. sus. | ** | ** | ** | ** | * | ns | Ns | ns | ** | |||
| Cv% | 39.8 | 70.0 | 21.1 | 25.5 | 16.3 | 7.4 | 6.7 | 11.4 | 29.7 |
Scores are based on mildew severity ratings of 10 plants per entry per season for three consecutive years made during October–November.
Disease scores are back transformations of arcsine [√(x/100)]. DSI was estimated from the obtained scores of 60-day-old plants and grading was done on the basis of DSI values using the 10-point scale of Horsfall–Barrett (Horsfall and Cowling 1978). Area under a disease progression curve was estimated from four scores of disease reactions made between 30 and 60 days per season. Leaves with mean DSI ≤ 12.0 and/or AUDPC ≤ 130.0 and ≥25.0 and/or ≥200.0 were considered resistant and susceptible, respectively.
aDisease severity index; baccumulative area under disease progression; cstomatal index; dstomatal density (no. mm−2); estomatal size (µm2); fabaxial and gadaxial cuticle thicknesses (µm); hleaf thickness (µm); itrichome density (no. mm−2).
– and spp. = unknown accession number and species, respectively.
*, ** and ns indicate significance at P < 0.05, P < 0.01 and non-significant, respectively.
Anatomical data are means of five observations per microscopic field (n= 5) for three consecutive seasons during October–November spanning 3 years.
Stomatal index and density, and trichome features, were significantly correlated with disease susceptibility (Table 3) and showed closely similar relationships with the DSI and AUDPC indices used for disease reaction measurements. Trichome density was negatively correlated with DSI (r = −0.809; P ≤0.01) and AUDPC (r = −0.801; P ≤0.01), while SI and SD were positively correlated with DSI (r = 0.576 and 0.624; P ≤0.01) and AUDPC (r = 0.561 and 0.651; P ≤0.01), respectively. A moderately positive correlation of SA and LT with DSI (r = 0.281 and 0.260; P ≤0.05) was observed. However, such correlations cannot be generalized since they showed a non-significant relationship with AUDPC. Moreover, the foliar anatomical features abaxial epidermal thickness (ETb) and adaxial epidermal thickness (ETd) showed non-significant relationships with both qualitative and quantitative powdery mildew reactions.
Table 3.
Pearson's correlation coefficient (r) between powdery mildew DSI or accumulative disease progression and seven micro-morphological characteristics of mulberry leaves. Thirty mulberry lines were evaluated under field conditions at Berhampore, West Bengal, India, over 3 years.
| SIa | SAb | SDc | CTbd | CTde | LTf | TDg | |
|---|---|---|---|---|---|---|---|
| DSIh | 0.576** | −0.281** | 0.624** | 0.051ns | 0.014ns | −0.260* | −0.809** |
| AUDPCi | 0.561** | −0.280* | 0.615** | 0.094ns | 0.005ns | −0.255ns | −0.801** |
** and * indicate significance at P< 0.01 and P< 0.05, respectively.
astomatal index; bstomatal area (µm2); cstomatal density (no. mm−2); dabaxial and eadaxial cuticle thicknesses (µm); fleaf thickness (µm); gtrichome density (no. mm−2); hdisease severity index; iaccumulative area under disease progression.
The strong association of SD, SI and TD with both disease responsiveness parameters was partitioned further through hierarchical cluster analysis. The clustering did not differ significantly between DSI and AUDPC. Because of the relatively higher correlations of DSI with SD, SI and TD (Table 3), we chose to present the DSI-based cluster result only (Fig. 1). The entries were grouped into two major clusters (A and B) with a wide linkage distance of 610.5. The sub-cluster A was further divided into two sub-groups (A1 and A2). Eight out of the total of 15 resistant germplasm lines were partitioned into A1 and A2 sub-groups. Two germplasm lines (‘Vietnam-2’ and ‘Zimbabwe-3’) in A1 had relatively less SD than the other six lines of the major cluster A. Cluster B represented 22 lines divided into five sub-groups (B1–B5). The sub-groups B1 and B2 included seven resistant germplasm lines with similar SD, SI and TD values to susceptible ‘RFS-135’, ‘Mother graft’, ‘Shrim-5’ and ‘Mizuzawa’. The remaining three sub-groups (B3–B5) represented 73 % of susceptible germplasm.
Fig. 1.
Dendrogram of 30 mulberry germplasm lines with contrasting degrees of susceptibility to powdery mildew. The relationships were obtained by analysing DSI, AUDPCs, SD, stomatal intensity and TD values during powdery mildew infection.
Discussion
Numerous constitutional defence mechanisms against powdery mildew infection have been demonstrated (Prats et al. 2006; Hockelhoven 2007). However, most studies have been concerned with the interaction of ectoparasitic powdery mildew pathogens with compatible hosts (Genre and Bonfante 2007; Fernandez-Aparicio et al. 2009). Our study provides, for the first time in mulberry, a characterization of the variability of leaf micro-morphological parameters in relation to powdery mildew infection. We used DSI and disease progression curves (AUDPC) to assess differences in powdery mildew susceptibility. These parameters are widely used for assessment of powdery mildew disease reactions (Lipps and Madden 1989; Campbell and Madden 1990). The consistent results across 3 years indicated a strong genetic component and a smaller environmental influence on mulberry powdery mildew resistance. Our results are compatible with those of Lillemo et al. (2006), where no influence of environmental (=years) variation affected grossly the level of resistance to powdery mildew in bread wheat progeny.
Our present study indicates that there is substantial germplasm variability in the micro-morphological characters we assessed. It also indicates that environmental (=years) variability was important for SI, SD and TD with a magnitude that warrants further examination. In other plants, a role for foliar micro-morphological traits connected with stomata, epidermis and trichomes in the resistance to biotrophic pathogens is widely acknowledged (Lake and Wade 2009). In our study, five out of seven micro-morphological parameters showed significant association with DSI and three with the disease progression curve. These findings are made the more persuasive by being obtained under the natural powdery mildew infection pressures of the field. They suggest a key role as constitutive barriers against the disease in mulberry. In general, the association with disease reactions was more pronounced for stomatal features and TD than for SA or LT. Although some lines in the mildew-susceptible germplasm (e.g. RFS-135, Mother graft, Shrim-5 and Mizuzawa) have a smaller SD, the number of stomata per unit area of leaf surface and SI were positively correlated (P ≤0.01) with powdery mildew resistance. Unlike Blumeria spp. and Uncinula spp., the mycelium of Phyllactinia spp. is hemi-endophytic (Braun 1987) and the hyphae of P. guttata enter the leaf mainly through stomata (Klein et al. 1998). There are some significant genotypic effects of stomatal frequency on penetration by powdery mildew pathogens (Lima et al. 2010). Our findings corroborate the view that the mechanical obstacles of epidermal thickness and waxy deposition could readily be bypassed during haustorial penetration when the surface porosity of the leaves is high (Gabler et al. 2003). We confirm that, in mulberry, resistance to powdery mildew infection increases with decreasing SD and stomatal intensity, with an opposite correlation between SA and DSI. In rust fungi, the emerging germ tubes adhere first to the leaf surface; subsequently, they grow and encounter stomata through directional growth (Wynn and Staples 1981), which in turn triggers appressorium formation (Anker and Niks 2001). Directional growth of the germ tube and formation of appressorium are controlled by the stimuli originating from the host (Hoch and Staples 1987). Excessive wax covering at the stomatal guard cell and epicuticular region obscure brown rust and powdery mildew germlings to recognize specific site(s) that normally triggers appressorium formation (Rubiales et al. 1996; Vaz Patto and Niks 2001). The epicuticular wax crystal pattern has been implicated either in directing or in disorienting germ tube growth across leaf surfaces (Jenks and Ashworth 1999; Rubiales et al. 2001). Typically, a germ tube length of 50–60 µm is required to reach the nearest stoma, leaving the germling sufficient energy to go on to infect the plant (Rubiales and Niks 1996). However, the Phyllactinia germ tube length needed to penetrate the plant surface through stomata remains unresolved. Moreover, extensive wax covering of the stomatal guard cells presumably decreases optimum gas exchange through the stomata required for pathogen penetration (Vaz Patto et al. 2003). In mulberry, stomata are randomly scattered over the abaxial leaf surface (Tikader and Rao 2002). In Lolium spp., Hordeum chilense and Pisum sativum, abaxial leaf surfaces showed more penetration resistance to leaf rust and powdery mildew conidia, respectively, than adaxial epidermal cells (Carver et al. 1990; Rubiales and Carver 2000; Gniwotta et al. 2005). The composition of abaxial and adaxial wax is different. The adaxial wax was characterized by a very high amount of primary alcohols, while abaxial wax consisted mainly of alkenes (Gniwotta et al. 2005). Variations of cuticular wax (Mamrutha et al. 2010) and secondary plant constituents like phenolics and flavonoids (Song et al. 2009) are also marked among mulberry accessions. A differential exudation of phenolic compound coumarins (like scopolin, ayapin and scopoletin) in the leaf surface of sunflower genotypes prevents rust germ tube growth and appressorium differentiation (Prats et al. 2007). Therefore, in addition to leaf architectural barriers such as epicuticular and/or stomatal guard cell wax content, the possibility of a higher exudation of coumarins onto the leaf surface of resistant lines of mulberry cannot be overruled. Our finding of a significantly smaller SD in powdery mildew-resistant lines compared with the susceptible group supports the possibility that germ tubes could also form misplaced appressoria that are too far from the nearest stoma. This situation has been reported in the case of the faba bean–rust interaction (Sillero and Rubiales 2002). A positive correlation of leaf rust resistance with SD on the abaxial leaf epidermis was reported in H. chilense lines and suggested that it might be a provision to compensate for the presumed less efficient gas change by wax-covered stomata (Rubiales and Niks 1996; Vaz Patto et al. 2001). But the exact implication of SD in Phyllactinia resistance in mulberry would be an area of further study in future. Nonetheless, our observations strongly suggest that stomatal traits can be used effectively in mulberry breeding programmes to predict powdery mildew resistance.
The contrast in TDs between the powdery mildew-resistant and mildew-susceptible germplasm groups was marked. Further, a strong negative correlation between TD and the AUDPC was also established. Our results support those of Shaik (1985), who found that resistance to Uromyces appendiculatus infection in beans was attributable to the physical effect of densely packed trichomes. However, the exact role of trichomes in preventing Phyllactinia spore penetration into the host leaf is still unknown. It has been reported that the conidia of an ectoparasitic powdery mildew pathogen need a liquid droplet to erode the cuticle immediately after contact (Mendgen 1996). From the work of Kortekamp and Zyprian (1999), it appears that an increased number of hydrophobic pubescences (such as trichomes) may repel water from the leaf surfaces, thus preventing successful penetration. Alternatively, a high trichome number may simply reduce the frequency of germ tube contact points that can lead to penetration (Niks and Rubiales 2002).
Both ETb and ETd and LT were very similar in most of the lines we studied. However, variation in cuticle thickness of both adaxial and abaxial surfaces was statistically significant across all 30 lines, but there was no clear difference between susceptible and tolerant groups. Our findings support previous work with Uncinula nector resistance in grape berries by Ficke et al. (2004), which showed only a weak relationship with cuticle thicknesses and disagree with the report of a positive correlation of cuticle thickness of various grape cultivars resistance to powdery mildew (Heintz and Blaich 1990). The exact reason for the discrepancy in the correlation between LT or SA and disease prevalence is uncertain. However, Phyllactinia, being a hemi-endophyte, penetrates the leaf surface through stoma; therefore, cuticle thickness might have little role in penetration resistance. Overall, our results indicate that resistance to powdery mildew is not strongly associated with cuticle thickness and only weakly associated with LT and the area of individual stoma.
The cluster analysis showed good agreement between the DSI and SD, SI and TD. However, three resistant lines (‘Kenmochi’, ‘M. laevigata’ and ‘Multicaulis’) did not reveal a sharp demarcation with four other susceptible lines of sub-group B2. The lack of tight clustering of these three lines may indicate that evaluation of other features is necessary to establish a more complete phylogenetic relationship among powdery mildew-responsive accessions.
It seems, for the first time, that an alternative ‘avoidance’ or pre-penetration mechanism, which operates after the contact of parasite on the host epidermal cell (Rubiales and Niks 1992; Vaz Patto et al. 2009) is apparent in mulberry–powdery mildew interaction. In several species, the pre-penetration resistance is often superimposed onto later-acting post-penetration hypersensitive resistance (Niks and Rubiales 2002). Contrary to relatively less durable hypersensitive resistance, pre-penetration avoidance is quantitative trait loci in nature, more durable and therefore has a greater value in breeding work (Vaz Patto et al. 2003).
Conclusion and forward look
Highly significant and similarly strong correlations were found between the prevalence of powdery mildew and three micro-morphological parameters (SI, SD and TD) among 30 lines of mulberry with known susceptibility or resistance to the pathogen. The links appeared causal and may be related to the level of successful spore penetration of the leaf. These three micro-morphological traits are thus of potential value when selecting for powdery mildew resistance from progeny or from collected material used in mulberry breeding programmes.
Source of funding
Research in this paper was supported by a grant from the Department of Biotechnology, Government of India (Grant No. BT/PR 5224/PBD 19/117).
Contributions by the authors
All the authors contributed to a similar extent to the experimental work. S.C. prepared the manuscript.
Conflict of interest statement
None declared.
Acknowledgements
Mr D. Biswas gave excellent technical and field assistance.
References
- Anker C, Niks RE. Prehaustorial resistance to the wheat leaf rust fungus, Puccinia triticina, in Triticum monococcum (s.s) Euphytica. 2001;117:209–215. [Google Scholar]
- Banerjee R, Chakraborty SP, Das BK. Morpho-anatomical traits and preliminary screening of mulberry (Morus spp.) genotypes for moisture stress condition. Indian Journal of Genetics. 2006;66:134–136. [Google Scholar]
- Berdahl JD, Mayland HF, Assay KH, Jefferson PG. Variation in agronomic and morphological traits among Russian wild rye accessions. Crop Science. 1999;39:1890–1895. [Google Scholar]
- Biasiolo M, DaCanal MT, Tornadore N. Micromorphological characterization of ten mulberry cultivars (Morus spp.) Economic Botany. 2004;9:639–646. [Google Scholar]
- Braun E. A monography of the Erysiphales (powdery mildews) Berlin: J. Cramer; 1987. pp. 1–700. [Google Scholar]
- Braun U, Cook RTA, Inman AJ, Shin HD. The taxonomy of powdery mildew fungi. In: Belanger RR, Bushnell WR, Dik AJ, Carver TLW, editors. The powdery mildews: a comprehensive treatise. St Paul, MN: ASP Press; 2002. pp. 13–55. [Google Scholar]
- Brown JKM. Comparative genetics of avirulence and fungicide resistance in the powdery mildew fungi. In: Belanger RR, Bushnell WR, Dik AJ, Carver TLW, editors. The powdery mildews: a comprehensive treatise. St Paul, MN: ASP Press; 2002. pp. 56–65. [Google Scholar]
- Campbell CL, Madden LV. Introduction to plant epidemiology. New York: John Wiley & Sons; 1990. [Google Scholar]
- Carver TWL, Thomas BJ, Ingerson-Morris SM, Roderick HW. The role of abaxial leaf surface waxes of Lolium spp. in resistance to Erysiphe graminis. Plant Pathology. 1990;39:573–583. [Google Scholar]
- Chattopadhyay S, Ali KA, Doss SG, Das NK, Aggarwal RK, Bandopadhyay TK, Sarkar A, Bajpai AK. Evaluation of mulberry germplasm for resistance to powdery mildew in the field and greenhouse. Journal of General Plant Pathology. 2010;76:87–93. [Google Scholar]
- Commenil P, Brunet L, Audran JC. The development of the grape berry cuticle in relation to susceptibility to bunch rot disease. Journal of Experimental Botany. 1997;48:1599–1607. [Google Scholar]
- Felle HH, Herrmann A, Hanstein S, Huckelhoven R, Kogel KH. Apoplastic pH signaling in barley leaves attacked by powdery mildew fungus Blumeria graminis f.sp. hordei. Molecular Plant-Microbe Interactions. 2004;17:118–123. doi: 10.1094/MPMI.2004.17.1.118. [DOI] [PubMed] [Google Scholar]
- Fernandez-Aparicio M, Prats E, Emersan AA, Rubiales D. Characterization of resistance mechanism to powdery mildew (Erysiphe betae) in beet (Beta vulguris) Phytopathology. 2009;99:385–389. doi: 10.1094/PHYTO-99-4-0385. [DOI] [PubMed] [Google Scholar]
- Ferris R, Taylor G. Stomatal characteristics of four native herbs following exposure to elevated CO2. Annals of Botany. 1994;73:477. [Google Scholar]
- Ficke A, Gadoury DM, Seem RC, Godfrey D, Dry IB. Host barriers and responses to Uncinula nector in developing grape berries. Phytopathology. 2004;94:438–445. doi: 10.1094/PHYTO.2004.94.5.438. [DOI] [PubMed] [Google Scholar]
- Gabler FM, Smilanick JL, Mansour M, ramming DW, Mackey BE. Correlations of morphological, anatomical and chemical features of grape berries with resistance to Botrytis cinerea. Phytopathology. 2003;93:1263–1273. doi: 10.1094/PHYTO.2003.93.10.1263. [DOI] [PubMed] [Google Scholar]
- Genre A, Bonfante P. Check-in procedure for plant cell entry by biotrophic microbes. Molecular Plant-Microbe Interactions. 2007;9:1023–1030. doi: 10.1094/MPMI-20-9-1023. [DOI] [PubMed] [Google Scholar]
- Glawe DA. The powdery mildews: a review of the world's most familiar (yet poorly known) plant pathogens. Annual Review of Phytopathology. 2008;46:27–51. doi: 10.1146/annurev.phyto.46.081407.104740. [DOI] [PubMed] [Google Scholar]
- Gniwotta F, Vogg G, Gartmann V, Carver TLW, Riederer M, Jetter R. What do microbes encounter at the plant surface? Chemical composition of pea leaf cuticular waxes. Plant Physiology. 2005;139:519–530. doi: 10.1104/pp.104.053579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez K, Gomez AA. Statistical procedure for agricultural research. New York: John Wiley & Sons; 1984. [Google Scholar]
- Govindaiah Gupta VP. Foliar disease of mulberry and their management. In: Sampath J, editor. Mulberry crop protection. Bangalore, India: Central Silk Board; 2005. pp. 145–177. [Google Scholar]
- Gupta VP. Diseases of mulberry and their management. In: Srivastava MK, editor. Plant pathology. Jaipur, India: Pointer Publishers; 2001. pp. 130–164. [Google Scholar]
- Heintz C, Blaich R. Ultrastructural and histochemical studies on interactions between Vitis vinifera L. and Uncinula necator (Schw.) Burr. New Phytologist. 1990;115:107–117. [Google Scholar]
- Hoch HC, Staples RC. Structural and chemical changes among the rust fungi during appressorium development. Annual Review of Phytopathology. 1987;25:231–247. [Google Scholar]
- Hockelhoven R. Cell wall associated mechanisms of disease resistance and susceptibility. Annual Review of Phytopathology. 2007;45:101–127. doi: 10.1146/annurev.phyto.45.062806.094325. [DOI] [PubMed] [Google Scholar]
- Horsfall JG, Cowling EB. Pathometry: the measurement of plant disease. In: Horsfall JG, Cowling EB, editors. Plant disease. New York: Academic Press; 1978. pp. 120–136. [Google Scholar]
- Itoi S, Nozu M, Kamachi T, Onuki M, Kumomura Y. Scanning electrion microscopy of overwintering and circumscissile dehiscence of cleistothecia of Phyllactinia moricola. Annals of Phytopathological Society of Japan. 1982;48:324–329. [Google Scholar]
- Jenks MA, Ashworth EN. Plant epicuticular waxes: function, production and genetics. Horticultural Reviews. 1999;23:1–69. [Google Scholar]
- Kim HS, Hartman GL, Manandhar JB, Graef GL, Steadman JR, Dier BW. Reaction of soybean cultivars to Sclerotia stem rots in field, greenhouse and laboratory evaluations. Crop Science. 2000;40:665–669. [Google Scholar]
- Klein LA, Windham MT, Trigiano RN. Natural occurrence of Microsphaera pulchra and Phyllactinia guttata on two Cornus species. Plant Disease. 1998;82:383–385. doi: 10.1094/PDIS.1998.82.4.383. [DOI] [PubMed] [Google Scholar]
- Kolkman JD, Kelly JD. Agronomic traits affecting resistance to white mold in common bean. Crop Science. 2002;42:693–699. [Google Scholar]
- Kortekamp A, Zyprian E. Leaf hairs as a basic protective barrier against downy mildew of grape. Journal of Phytopathology. 1999;147:453–459. [Google Scholar]
- Lake JA, Wade RN. Plant pathogen interactions and elevated CO2: morphological changes in favour of pathogens. Journal of Experimental Botany. 2009;60:3123–3131. doi: 10.1093/jxb/erp147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillemo M, Skinnes H, Singh RP, Ginkel MV. Genetic analysis of partial resistance to powdery mildew in bread wheat line Saar. Plant Disease. 2006;90:225–228. doi: 10.1094/PD-90-0225. [DOI] [PubMed] [Google Scholar]
- Lima P, Lopes CA, CafeFilho AC. Stomatal patterns of Capsicum genotypes resistant or susceptible to Oidlopsis haplophylli. Summa Phytopathologia. 2010;36:25–29. [Google Scholar]
- Lipps PE, Madden LV. Assessment methods of determining powdery mildew severity in relation to grain yield of winter wheat cultivar in Ohio. Phytopathology. 1989;79:462–470. [Google Scholar]
- Mamrutha HM, Mogili T, Jhansi Lakshmi K, Rama N, Kosma D, Udaya Kumar M, Jenks MA, Nataraja KN. Leaf cuticular wax amount and crystal morphology regulate post harvest water loss in mulberry (Morus species) Plant Physiology and Biochemistry. 2010;48:690–695. doi: 10.1016/j.plaphy.2010.04.007. [DOI] [PubMed] [Google Scholar]
- Manimegalai S, Chandramohan N. Leaf quality of mildew affected leaves and their effect on mortality and economic characters of silkworm, Bombyx mori L. Sericologia. 2007;47:87–92. [Google Scholar]
- Martin C, Glover BJ. Functional aspects of cell patterning in aerial epidermis. Current Opinion in Plant Biology. 2007;10:70–78. doi: 10.1016/j.pbi.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Mendgen K. Fungal attachment and penetration. In: Kerstiens G, editor. Plant cuticles. Oxford: BIOS; 1996. pp. 175–188. [Google Scholar]
- Mishra MK. Stomatal characteristics at different ploidy levels in Coffea L. Annals of Botany. 1997;80:689–692. [Google Scholar]
- Mmbaga MT, Steadman JR, Roberts JJ. Interaction of bean leaf pubescence with rust uredinospore deposition and subsequent infection density. Annals of Applied Biology. 1994;125:243–254. [Google Scholar]
- Niks RE, Rubiales D. Potentially durable resistance mechanisms in plants to specialized fungal pathogens. Euphytica. 2002;124:201–216. [Google Scholar]
- O'Connell RJ, Panstruga R. Tete a tete inside a plant cell: establishing compatibility between plants and biotrophic fungi and oomycetes. New Phytologist. 2006;171:699–713. doi: 10.1111/j.1469-8137.2006.01829.x. [DOI] [PubMed] [Google Scholar]
- Prats E, Gay AP, Mur LAJ, Thomas BJ, Carver TLW. Stomatal lock open, a consequence of epidermal cell death, follows transient suppression of stomatal opening in barley attacked by Blumeria graminis. Journal of Experimental Botany. 2006;57:2211–2226. doi: 10.1093/jxb/erj186. [DOI] [PubMed] [Google Scholar]
- Prats E, Llamas MJ, Jorrin J, Rubiales D. Constitutive coumarins accumulation on sunflower leaf surfaces prevents rust germ tube growth and appressorium differentiation. Crop Science. 2007;47:1119–1124. [Google Scholar]
- Rubiales D, Carver TWL. Cellular responses of Hordeum chilense genotypes to inappropriate formae specialis of the cereals powdery mildew fungus. Canadian Journal of Botany. 2000;78:1561–1570. [Google Scholar]
- Rubiales D, Niks RE. Low appressorium formation by rust fungi on Hordeum chilense leaves. Phytopathology. 1992;82:1007–1012. [Google Scholar]
- Rubiales D, Niks RE. Avoidance of rust infection by some genotypes of Hordeum chilense due to their relative inability to induce the formation of appressoria. Physiological and Molecular Plant Pathology. 1996;49:89–101. [Google Scholar]
- Rubiales D, Ramirez MC, Niks RE. Avoidance of leaf rust fungi in wild relatives of cultivated cereals. Euphytica. 1996;87:1–6. [Google Scholar]
- Rubiales D, Ramirez MC, Carver TLW, Niks RE. Abnormal germling development by brown rust and powdery mildew on cer barley mutants. Hereditas. 2001;135:271–276. doi: 10.1111/j.1601-5223.2001.t01-1-00271.x. [DOI] [PubMed] [Google Scholar]
- Shaik M. Race non-specific resistance in bean cultivars to races of Uromyces appendiculatus var. appendiculatus and its correlation with leaf epidermal characters. Phytopathology. 1985;75:478–481. [Google Scholar]
- Sharma A, Sharma R, Machii H. Assessment of genetic diversity in a Morus germplasm using fluorescence based AFLP markers. Theoretical and Applied Genetics. 2000;101:1049–1055. [Google Scholar]
- Sillero JC, Rubiales D. Histological characterization of resistance to Uromyces viciae-fabae in faba bean. Phytopathology. 2002;92:294–299. doi: 10.1094/PHYTO.2002.92.3.294. [DOI] [PubMed] [Google Scholar]
- Smith PH, Foster EM, Boyd LA, Brown JKM. The early development of Erysiphe pisi on Pisum sativum L. Plant Pathology. 1996;45:302–309. [Google Scholar]
- Song W, Wang H-J, Bucheli P, Zhang P-F, Wei D-Z, Lu Y-H. Phytochemical profiles of different mulberry (Morus sp.) species from China. Agricultural and Food Chemistry. 2009;57:9133–9140. doi: 10.1021/jf9022228. [DOI] [PubMed] [Google Scholar]
- Takamatsu S, Inagaki M, Niinomi S, Khodaparast SA, Shin HD, Grigaliunaite B, Havrylenko M. Comprehensive molecular phylogenetic analysis and evalution of the genus Phyllactinia (Ascomycota: Erysiphales) and its allied genera. Mycological Research. 2008;111:299–315. doi: 10.1016/j.mycres.2007.11.014. [DOI] [PubMed] [Google Scholar]
- Tikader A, Rao AA. Phenotypic variation in mulberry (Morus spp.) germplasm. Sericologia. 2002;42:221–233. [Google Scholar]
- Valverde PL, Fornoni J, Nunez-Farfan J. Defensive role of leaf trichomes in resistance to herbivorous insects in Datura stramonium. Journal of Evolution Biology. 2001;14:424–432. [Google Scholar]
- Vanacker H, Carver TLW, Foyer CH. Early H2O2 accumulation in mesophyll cells leas to induction of glutathione during the hyper-sensitive response in the barley powdery mildew interaction. Plant Physiology. 2000;123:1289–1300. doi: 10.1104/pp.123.4.1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaz Patto MC, Niks RE. Leaf wax layer may prevent appressorium differentiation but does not influence orientation of the leaf rust fungus Puccinia hordei on Hordeum chilense leaves. European Journal of Plant Pathology. 2001;107:795–803. [Google Scholar]
- Vaz Patto MC, Rubiales D, Martin A, Hernandez P, Lindhout P, Niks RE, Stam P. QTL mapping provides evidence for lack of association of the avoidance of leaf rust in Hordeum chilense with stomata density. Theoretical and Applied Genetics. 2003;106:1283–1292. doi: 10.1007/s00122-003-1195-2. [DOI] [PubMed] [Google Scholar]
- Vaz Patto MC, Fernandez-Aparicio M, Moral A, Rubiales D. Pre- and post-haustorial resistance to rust in Lathyrus cicera L. Euphytica. 2009;165:27–34. [Google Scholar]
- Vijayan K, Chakraborti SP, Ercisli S, Ghosh PD. NaCl induced morpho-biochemical and anatomical changes in mulberry (Morus spp.) Plant Growth Regulation. 2008;56:61–69. [Google Scholar]
- Wise RR, Cole GSC, Percy RG. A comparison of leaf anatomy in field grown Gossypium hirsutum and G. barbadense. Annals of Botany. 2000;86:731–738. [Google Scholar]
- Wynn WK. Appersorium formation over stomates by the bean rust fungus: response to a surface contact stimulus. Phytopathology. 1976;66:136–146. [Google Scholar]
- Wynn WK, Staples RC. Tropism of fungi in host recognition. In: Staples RC, Toenilsen GA, editors. Plant disease control: resistance and susceptibility. New York: John Wiley; 1981. pp. 45–69. [Google Scholar]
- Zeyen RJ, Carver TLW, Lyngkjaer MF. Epidermal cell papillae. In: Belanger RR, Bushnell WR, Dik AJ, Carver TLW, editors. The powdery mildews: a comprehensive treatise. St Paul, MN: ASP Press; 2002. pp. 107–125. [Google Scholar]