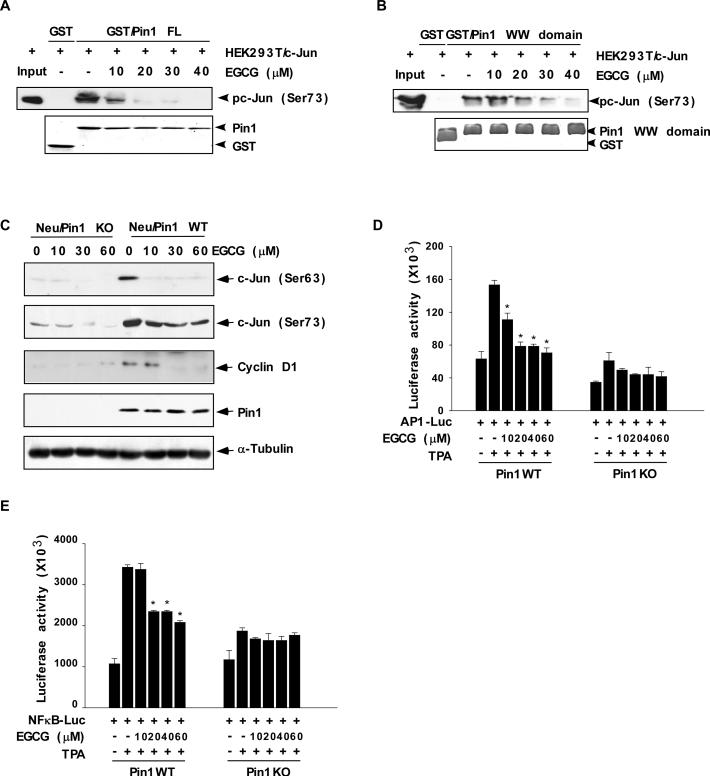
Fig. 4. EGCG inhibits the interaction of Pin1 with active c-Jun and attenuates TPA-induced AP-1 and TPA-induced NF-κB promoter activities.
(A) The effect of EGCG on GST/Pin1 full-length (FL) protein or (B) the GST/Pin1 WW domain and active c-Jun binding affinity. Lysates from HEK293T cells transfected with c-Jun were treated with different concentrations of EGCG and then subjected to a pull-down assay with GST, GST/Pin1 FL, or GST/Pin1 WW domain followed by immunoblotting with an antibody to detect phosphorylation of c-Jun (Ser73). Purified GST, GST/Pin1 FL, or GST/Pin WW domain abundance was visualized by Coomassie brilliant blue staining (bottom panels A, B). (C) The effect of EGCG on phosphorylation of c-Jun and cyclin D1 protein expression level in Neu/Pin1 WT and KO MEFs. Phosphorylation of c-Jun at Ser63 or Ser73 or cyclin D1 expression level was detected in lysates prepared from cells treated 24 h with various doses (0-30 μM) of EGCG. A specific Pin1 antibody was used to detect the abundance of Pin1 in the same samples. The firefly luciferase (D) AP-1- or (E) NF-κB reporter gene activity was assessed. For the reporter-gene assay, Pin1WT and Pin1KO MEFs were transfected with a plasmid mixture containing the (D) AP-1- or (E) NF-κB-luciferase reporter gene (100 ng) and the pRL-SV40 gene (10 ng) for normalization. At 24 h after transfection, cells were treated with various concentrations of EGCG (0, 10, 20, 40, or 60 μM) for 1 h before treatment with 20 ng/ml TPA for an additional 24 h. Data are shown as means ± S.D. of triplicate samples from 3 independent experiments. The asterisk (*) indicates a significant change relative to Pin1WT cells treated with only TPA (*, p < 0.005).