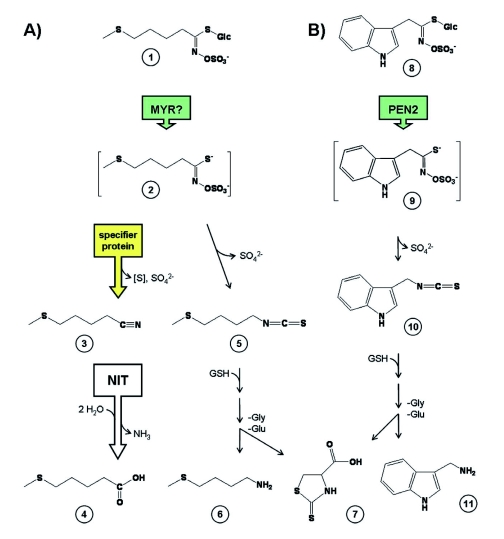
Figure 4.
Glucosinolate breakdown in intact tissue.
Shown are (A) two hypothetical pathways of glucosinolate turnover (exemplified with 4-methylthiobutylglucosinolate (1)) and (B) the PEN2-dependent breakdown pathway of indole glucosinolates induced upon pathogen attack (exemplified with indol-3-ylmethylglucosinolate (8)). In A), myrosinase-type enzymes (classical myrosinases or atypical myrosinases, MYR) hydrolyze (1) to the corresponding aglucone (2). The simple nitrile (3) formed if a specifier protein is present can be further converted by nitrilases (NIT) to a carboxylic acid (4). This pathway would release both core structure sulfur atoms (as sulfate and likely as elemental sulfur [S]) as well as the nitrogen (as ammonia). In the absence of specifier protein activity, the isothiocyanate (5) would be formed instead, which might further react to a glutathione-conjugate decomposing to the amide (6) and raphanusamic acid (7). In B), hydrolysis of indol-3-ylmethylglucosinolate (8) to the corresponding aglucone (9) catalyzed by the atypical myrosinase PEN2 leads to the formation of indol-3-ylmethylisothiocyanate (10) which appears to be conjugated to glutathione and subsequently broken down to raphanusamic acid and indol-3-ylmethylamine (11). PEN2-dependent breakdown of 4-methoxyindol-3-ylmethylglucosinolate (not shown in the figure as the breakdown products are still unknown) is required for pathogen resistance in Arabidopsis.