Abstract
More than 60 years ago, H.H. Flor proposed the “Gene-for-Gene” hypothesis, which described the genetic relationship between host plants and pathogens. In the decades that followed Flor's seminal work, our understanding of the plant-pathogen interaction has evolved into a sophisticated model, detailing the molecular genetic and biochemical processes that control host-range, disease resistance signaling and susceptibility. The interaction between plants and microbes is an intimate exchange of signals that has evolved for millennia, resulting in the modification and adaptation of pathogen virulence strategies and host recognition elements. In total, plants have evolved mechanisms to combat the ever-changing landscape of biotic interactions bombarding their environment, while in parallel, plant pathogens have co-evolved mechanisms to sense and adapt to these changes. On average, the typical plant is susceptible to attack by dozens of microbial pathogens, yet in most cases, remains resistant to many of these challenges. The sum of research in our field has revealed that these interactions are regulated by multiple layers of intimately linked signaling networks. As an evolved model of Flor's initial observations, the current paradigm in host-pathogen interactions is that pathogen effector molecules, in large part, drive the recognition, activation and subsequent physiological responses in plants that give rise to resistance and susceptibility. In this Chapter, we will discuss our current understanding of the association between plants and microbial pathogens, detailing the pressures placed on both host and microbe to either maintain disease resistance, or induce susceptibility and disease. From recognition to transcriptional reprogramming, we will review current data and literature that has advanced the classical model of the Gene-for-Gene hypothesis to our current understanding of basal and effector triggered immunity.
INTRODUCTION
Since the last The Arabidopsis Book chapter outlining the Arabidopsis-Pseudomonas syringae interaction (Katagiri et al., 2002), there have been a number of advances in our understanding of how plants perceive and respond to biotic stress. In this respect, Arabidopsis has continued to lead the way in these advances, both in regard to understanding host defenses, as well as uncovering pathogen virulence strategies. A plant's response to environmental pressures is guided by its ability to sense and process stimuli So too is a plant's ability to detect and respond to pathogen infection. In total, these processes are regulated in large part by the genetic and biochemical exchange between host and pathogen. In this Chapter, we will outline our current understanding of how plants and pathogens communicate through the balance of resistance and susceptibility. A “dance”, a “molecular arms race”, or simply survival, the interaction between a plant and pathogen represents a sophisticated interplay of genetic and biochemical processes, ultimately leading to the demise of either the host or the invader. Here, we will focus on the architecture of the plant immune response, highlighting the key advances in our understanding of host cell physiology, the activation of specific defense responses, and too, the evolution of strategies by the invading pathogen to shut down defense signaling in plants.
In a recent review by Alan Jones and colleagues (Jones et al., 2008), a parallel is drawn between research advances in humans and those that can be directly attributed to studies first conducted in the model plant Arabidopsis thaliana. For example, approximately 70% of the genes associated with the development of cancer(s) in humans have orthologs present in Arabidopsis. Furthermore, with respect to advances in research first undertaken in Arabidopsis and subsequently “translated” in human disease research, innate immune receptor identification in plants have made significant impacts in our understanding of disease signaling in humans; resistance proteins were first identified and characterized in Arabidopsis (ca. 1994) before their counterparts (e.g., NOD/CARD/CATERPILLAR) in humans (ca. 2000; Ting et al., 2006). Jones and colleagues cite additional examples where research findings in Arabidopsis have advanced the broader study of biology in humans, including research in the area of circadian rhythms (Ahmad and Cashmore, 1993), RNA silencing (Hamilton and Baulcombe, 1999) and G-protein signaling (e.g., Temple and Jones, 2007).
Disease and defense signaling in plants, as we will outline throughout this Chapter, is a complicated, highly regulated process, involving the coordinated signaling networks of both host and pathogen. In this regard, the development of model systems that are both tractable and translational have been critical to addressing the many facets of the host-pathogen interface. Below, we will give a broad overview of several of the processes that typify studies in the area of plant-pathogen interactions, and too, highlight their significance towards increasing our understanding of host defense signaling in response to pathogen infection.
a. Pseudomonas syringae
Pseudomonas syringae is a Gram-negative plant pathogenic bacterium that was commonly known to cause bacterial speck disease on tomato (Pedley and Martin, 2003; http://pseudomonas-syringae.org/). Towards developing the laboratory-based tools we now have at our disposal, several strains were identified in the 1980's that would infect Arabidopsis (Katagiri et al., 2002), giving birth to a new era in molecular plant pathology. Since the establishment of the Arabidopsis-Pseudomonas pathosystem, research has explored nearly all facets of the interaction, from epiphytic colonization of the leaf surface (Hirano and Upper, 2000), pathogen entry through, and manipulation of, stomata (Melotto et al., 2006), as well as the delivery of effectors (Lindeberg et al., 2009) and induction of cell death (Figure 1; reviewed in Kim et al., 2008). As a consequence of standard mechanisms of dispersal (i.e., rain splash, insects, animals, humans, etc.), P. syringae establishes itself on the surface of plants as an epiphyte, before gaining entry into the intercellular space (Hirano and Upper, 2000). Once inside the intercellular space, the pathogen employs a type III secretion system (T3SS) for the delivery of effector proteins directly into the host cell. In total, it is the action of these effectors that promote pathogenicity, shutting down critical host processes required to fight pathogen infection. Thus, the T3SS is essential for the development of disease symptoms and bacterial multiplication (reviewed in Lindeberg et al., 2009).
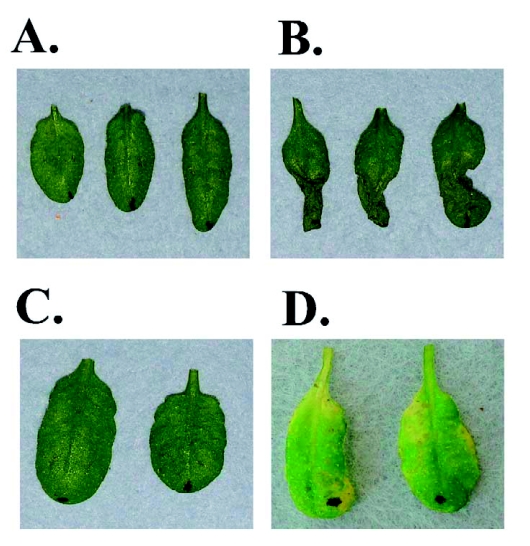
Figure 1:
The Arabidopsis thaliana-Pseudomonas syringae pathosystem.
A) Phenotype of the healthy Arabidopsis leaves.
B) Leaves undergoing the hypersensitive response (24 hpi).
C) Leaves inoculated with a non-disease eliciting P. syringae strain.
D) Leaf symptoms of the bacterial speck disease. hpi, hours post-inoculation.
In 2009, researchers in the field of plant-microbe interactions marked the 25th anniversary since the cloning of the first bacterial type III secreted effector protein. In the November 2009 issue of the journal Molecular Plant Pathology, Brian Staskawicz reflects on the advances in the field of molecular plant pathology since his lab's seminal discovery (Staskawicz et al., 1984; Staskawicz et al., 2001; Staskawicz, 2009). Since 1984, advances in the area of plant-pathogen interactions have shaped our understanding of microbial genetics and pathogenicity, as well as plant physiology and evolution (reviewed in Cui et al., 2009). Collectively, these bacterial proteins, called “effectors”, function to manipulate host cell processes for the purpose of enhancing infection and pathogen proliferation. While the function of the full suite of effector proteins remains unknown, what is known is that the complex genetic and biochemical interactions between pathogen effectors and their cognate host proteins evoke specific responses, that when recognized, elicit disease resistance, or when evaded, promote susceptibility.
b. Fungal and Oomycete Pathogens
Much like the bacterial virulence strategies described above, fungi and oomycete pathogens have also evolved mechanisms to infect and colonize plants. Beyond the presence of highly conserved pathogen components, known as Pathogen Associated Molecular Patterns (PAMPs), which are perceived by the host, fungal pathogens have the ability to stimulate the release of host cell wall molecules through the production of hydrolytic enzymes during host invasion. These molecules, termed DAMPs (Danger-Associated Molecular Patterns), can be recognized by the plant and subsequently activate the defense response (Matzinger, 2007; Denoux et al., 2008). In addition to secreted hydrolytic enzymes and toxins, fungal and oomycete pathogens also encode for a suite of effector proteins, putatively similar in function to their bacterial counterparts (reviewed in De Wit et al., 2009; Schornack et al., 2009). However, one of the major differences with regard to effector action between bacterial pathogens and fungal or oomycete pathogens lies in the delivery of the effectors themselves. While bacteria rely on the T3SS, fungal and oomycete pathogens do not utilize a T3SS, and must instead rely on other mechanisms for effector delivery. At present, the specific mechanism(s) required for fungal or oomycete effector delivery is unknown.
In general, fungal effectors fall into two groups: those that are secreted into the host apoplast, and those that are translocated into the host cells (De Wit et al., 2009). In fungal pathogens, the mechanism by which the effectors are translocated remains elusive, and in oomycetes, while the specific mechanism of translocation is unknown, a conserved motif has been identified as being sufficient for effector uptake by host cells (Whisson et al., 2007). In short, many of these cytoplasmic oomycete effectors consist of an N-terminal region involved in secretion and translocation, as well as a C-terminal domain possessing the biochemical activity of the effector itself (Morgan and Kamoun, 2007). In recent years, a signature motif at the N-terminus (i.e., Arg-X-Leu-Arg; RxLR) has been identified and characterized as a critical component that not only guides oomycete effector identification (i.e., bioinformatics), but is also a critical component in the function of these secreted proteins during host interactions (Whisson et al., 2007).
c. Non-host Systems and Disease Resistance
As we will discuss in more detail below, a pathogen's ability to colonize any given host is regulated in large part by its ability to avoid structural and preformed defenses, as well as abrogate or circumvent induced host-specific defenses. This begs the question: What are the initial responses by both plant and pathogen that determine host-specificity? Moreover, what differentiates host-specific from non-host interactions, and how is defense signaling regulated in each? To answer this question, research in the area of non-host resistance has revealed at least two layers of signaling: pre- and post-invasion disease resistance (reviewed in Mysore and Ryu, 2004). While most plants are resistant to most pathogens, the cellular and genetic responses that tip this balance in favor of the pathogen have been best characterized using non-adapted pathogens such as the cucurbit powdery mildew pathogen Golovinomyces cichoracearum, and Blumeria graminis, a powdery mildew of the grasses. As host-specific pathogens, pathogen entry is effective, with a penetration rate of approximately 70% on their respective hosts (reviewed in Lipka et al., 2008). However, when Arabidopsis plants are inoculated, this rate falls dramatically. Herein lies the premise for the further characterization and identification of components required for pathogen entry and host-mediated responses to infection.
To identify and define the host mechanisms associated with resistance to non-adapted pathogens, initial work began with an extensive mutagenesis screen to identify host factors responsible for abrogating pathogen entry (Collins et al., 2003). To this end, early work demonstrated that plants attempt to prevent penetration by fungal pathogens through the formation of cell wall appositions termed papillae (Aist and Bushnell, 1991). To explore penetration resistance, ethyl methanesulfonate (EMS) mutagenized Arabidopsis populations were screened for increased penetration by the non-adapted powdery mildew fungus Blumeria graminis f. sp. hordei (Collins et al., 2003; Lipka et al., 2005, Stein et al., 2006). Three penetration, or PEN, mutants have been characterized. PEN1 encodes for an Arabidopsis syntaxin, which is predicted to function in the targeted trafficking of secretory vesicles to sites of papillae formation in response to attempted fungal pathogen penetration (Collins et al., 2003). PEN2 and PEN3 have been shown to function in the same pathway (Stein et al., 2006), also at sites of attempted fungal penetration. Subsequent work has gone on to show that PEN2 is a myrosinase functioning in the glucosinolate pathway (Clay et al., 2009), while PEN3 is an ABC (ATP-binding cassette) transporter (Stein et al., 2006) thought to be involved in the efflux of antimicrobial compounds to sites of attempted pathogen penetration. In total, these observations demonstrate that preformed responses are critical to the ability of the plant to resist penetration. As such, the suite of pre-invasive defense responses present in plants is sufficient to limit non-host pathogen entry; these include generalized responses such the deposition of callose at the site of attempted pathogen entry (Aits and Bushnell, 1991; reviewed in Hématy et al., 2009), as well as a dynamic reorganization of the host actin cytoskeleton. These responses coincide with increased cellular trafficking of organelles and defense signaling molecules to the site of infection. As will be a common theme throughout this Chapter, considerable overlap in defense signaling exists both in the initial perception and activation of cell signaling to numerous pathogen species, as well as critical defense signaling nodes associated with signal transduction amplification and the onset of disease resistance. Interestingly, however, the PEN mutations have not been reported to compromise resistance to bacterial pathogens, such as P. syringae (Lipka et al., 2008). These results suggest that restriction of host range to phytopathogens is regulated by additional other mechanisms.
HOST ARCHITECTURE AND PHYSIOLOGY
The plant cell is a remarkable evolutionary product of chemical, mechanical and electrical engineering. The structural capacity of the plant cell to resist mechanical forces from biotic and abiotic pressures is evidenced through the strength and elasticity of the cell wall (reviewed in Hématy et al., 2009). As discussed below, the cell wall can serve as a passive barrier to pathogen entry, as well as the site of first contact between host and pathogen. Serving in a more dynamic capacity, plants have the ability to actively reinforce their cells walls in response to a pathogen, such as in attempted fungal penetration, by the deposition of callose at sites of infection (Aits and Bushnell, 1991) as discussed previously.
Preformed Defenses
The leaf surface presents a formidable barrier to pathogen colonization and entry. Studded with trichomes, the leaf's waxy surface provides an unwelcoming environment from which pathogens must attempt to colonize and gain entry into the host. The outermost layers of the leaf epidermis consist of a modification to the cell wall known as a cuticle (Nawrath, 2006), which is comprised of cutin and waxes secreted onto the exterior surface of the cell (Jeffree, 2006). In addition to serving as a barrier to pathogen entry, the cutin is indispensible for the prevention of water loss from the leaf surface (Aharoni et al., 2004). There is growing evidence for the cuticle as a major player in Arabidopsis resistance to a wide variety of pathogen types from the bacterial pathogen P. syringae to the fungal pathogen Botrytis cinerea (reviewed in Reina-Pinto and Yephremov, 2009).
Once a bacterial pathogen gains entry to the leaf apoplast, it must still interact with the host cytoplasm in order to acquire nutrients; thus, the basic plant cell wall still proves a substantial barrier to pathogen entry. The rigid cell wall can therefore be viewed as a major constituent of resistance to non-adapted pathogens (reviewed in Hématy et al., 2009). While the physical barriers to pathogen entry are substantial, additional preformed defenses, such as chemical defenses, play ubiquitous roles in basal defense responses against pathogen infection. Among the best-characterized modes of chemical defenses are the phytoanticipins, which represent a diverse group of antimicrobial compounds present in the host before pathogen infection (VanEtten et al., 1994). This is in contrast to phytoalexins, which are by definition formed in response to pathogen infection, such as the well-characterized Arabidopsis phytoalexin, camalexin (reviewed in Glawischnig, 2007). Camalexin, 3-Thiazol-2′yl-indole, was originally isolated from leaves of the crucifer Camelina sativa infected with Alternaria brassicae (Browne et al. 1991), and was subsequently identified in Arabidopsis challenged with P. syringae (Tsuji et al., 1992), and its production was found to be induced by a wide range of stress conditions (reviewed in Glawischnig, 2007). However, production levels (and concentration) vary greatly within and among associated stresses. As is the case with all pathogen-induced defense responses in plants, phytoalexins are not an impenetrable barrier to infection and subsequent proliferation. In support of this, multiple pathogens have been identified that are able to tolerate camalexin production in Arabidopsis through a variety of mechanisms. Isolates of the root rot fungus Rhizoctonia solani have the ability to degrade camalexin through the 5-hydroxlyation of its indole ring, or by the formation of an oxazoline derivative (Pedras and Khan, 1997, 2000). In the case of the fungal pathogen B. cinerea, both resistant and sensitive isolates have been identified (Kliebenstein et al., 2005). This mechanism of resistance is mediated in B. cinerea through the activity of an ABC transporter, BcatrB, which acts as an efflux pump for removing camalexin from the cell (Stefanato et al., 2009).
Hormones and Defense Signaling
Extensive research has unraveled the intimate link between plant development, responses to the environment and pathogen perception. Through all of this, the role of plant hormones has been revealed as a central, key component in not only regulating defense signaling responses within infected cells, but also as a mediator of systemic signaling (reviewed in Spoel and Dong, 2008). At a primary level, plant hormones are responsible for the integration and processing of developmental and environmental cues. To this end, they are responsible not only for shaping the dynamic regulatory processes that control development, reproduction and death, but also priming the host cell for both biotic and abiotic stress responses. Of the major plant hormones, salicylic acid (SA), jasmonic acid (JA) and ethylene have been shown to play key roles in defense signaling in plants (reviewed in Bari and Jones, 2009).
It is widely known that pathogen infection affects plant development (Block et al., 2010; Chandra and Huff, 2010), and in large part, this effect is manifested through perturbations in hormone signaling within the host plant (Chen et al., 2007). As discussed above, pathogens have evolved elaborate mechanisms to colonize and infect their host; typically through the manipulation of host physiology by secreted pathogen effectors. During a typical infection, P. syringae delivers approximately 32 effector proteins inside its host (Lindeberg et al., 2009). Of these, one of the best characterized is AvrRpt2, a cysteine protease, whose catalytic activity sets into motion a series of defense signaling responses which have become hallmark tenants for the Gene-for-Gene and Guard Hypotheses. However, aside from AvrRpt2's well-established role in avirulence, studies investigating the manipulation of host physiology, and more specifically hormone signaling, have revealed an intimate link between pathogen effector action and hormone signaling. In 2007, Chen and colleagues (Chen et al., 2007) demonstrated a link between AvrRpt2-mediated defense signaling and the elicitation of host auxin biosynthesis. Phenotypically, plants expressing AvrRpt2 were found to be similar in stature to plants over-expressing auxin; plants have longer primary roots, increased lateral root formation and enhanced sensitivity to exogenously applied auxin (Sato and Yamamoto, 2008). One interesting finding of this study was the link between AvrRpt2 action within the host cell and hormone biosynthesis. In short, AvrRpt2-expressing plants were found to have elevated levels of free indole-3-acetic acid (IAA). The link between host defense, pathogen virulence and hormone perception was further supported as a consequence of enhanced disease symptom development (Chen et al., 2007).
In an example analogous to manipulation of auxin biosynthesis by pathogens, described above, recent evidence also suggests that SA inhibits pathogen growth by suppressing auxin signaling (Wang et al., 2007). Through the use of expression profiling, Wang and colleagues (2007) found that SA inhibits auxinmediated signaling, partially countering the pathogen's impact on hormone-associated defense signaling. This work showed that the SA analog BTH (benzothiadiazole-s-methyl ester) suppressed the expression of auxin responsive genes in an NON-EXPRESSOR of PR1 (NPR1)-dependent manner. In total, this work demonstrated the host plant's ability to antagonistically co-regulate multiple hormone signaling networks in response to pathogen infection, and with that, strengthens the hypothesis that plants may divert limited resources to defense-related processes at the expense of plant growth when attacked by a pathogen.
Endocytosis, Trafficking and Cellular Dynamics
In recent years, advances in imaging and cell biology technologies have made possible the observation of the dynamic responses to pathogen infection, such as increases in cellular trafficking, (re)-localization of proteins following pathogen perception, as well as reorganization of the actin cytoskeleton. In total, these collective works have not only enabled researchers to glimpse the cellular processes that are impacted during pathogen infection, but to also identify additional signaling components required for defense and disease resistance activation in plants. As we discussed above, the primary defense response in plants following pathogen perception is collectively referred to as PAMP-triggered immunity (PTI; Chisholm et al., 2006; Jones and Dangl, 2006). In this regard, the flagellin recognition receptor, FLS2, is a key component in both the initiation and amplification of basal defense responses in plants following pathogen perception (Figure 2A). In an eloquent series of experiments by Robatzek and colleagues (Robatzek et al., 2006), FLS2 was found to enter the endocytic pathway upon flg22 perception, resulting in the rapid accumulation of FLS2 in intracellular vesicles. In total, this series of experiments has led to a more complete understanding of receptor endocytosis in plants, and too, the regulatory network that follows PAMP perception leading to activation of PTI (reviewed in Irani and Russinova, 2009).
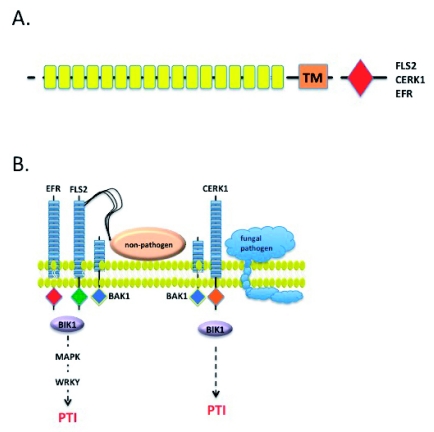
Figure 2:
Pathogen Associated Molecular Pattern Recognition and the Activation of PAMP-Triggered Immunity.
A) The PAMP receptors FLS2, CERK1 and EFR. Yellow boxes denote leucine-rich repeats (LRRs); CC, coiled-coil; NB, nucleotide-binding site; TIR, Toll-Interleukin-1 Receptor. Red diamond denotes kinase domain.
B) As the first layer of defense signaling in plants, PTI is activated via the recognition of conserved pathogen elicitors, generally referred to as PAMPs. Well-characterized PAMPs include flagellin, the bacterial elongation factor, EF-Tu, and the fungal cell wall component, chitin. Binding of PAMPs to their corresponding effectors (e.g., flagellin-FLS2; EF-Tu-EFR; chitin-CERK1) results in the activation of downstream defense signaling, via MAPK activation, resulting in the elicitation of immunity.
Once receptor-mediated endocytosis occurs, as in the case of flg22 perception via FLS2 described above, a plant's response to pathogen perception is further amplified by the intercellular trafficking of defense-associated compounds. Thus, from the standpoint of disease resistance, the host plant must mobilize defenseassociated components both to the site of infection, as well as within, and amongst, adjacent cells. From the standpoint of pathogen virulence, shutting down this response is key to continued infection and proliferation. None too surprising, changes in the host cell endomembrane system have also been observed during effector-triggered immunity (ETI; Chisholm et al., 2006; Jones and Dangl, 2006). For example, work from the laboratory of Sheng Yang He demonstrated that the secreted effector protein HopM1 from P. syringae localizes to plant endomembrane fractions (Nomura et al., 2006). With this information, an investigation into possible host targets revealed an association between HopM1 and the Arabidopsis protein MIN7 (i.e., AtMIN7). This work demonstrated that AtMIN7 encodes for an adenosine diphosphate ribosylation factor (ARF) guanine nucleotide exchange factor (GEF), further solidifying the link between HopM1 function and the regulation of vesicle trafficking during plant-pathogen interactions. Confirmation of these observations, using a pharmacological approach, Nomura and colleagues found that application of the fungal-derived antibiotic Brefeldin-A phenocopied the activity of HopM1; Brefeldin-A interferes with endomembrane protein transport from the Golgi apparatus to the endoplasmic reticulum. In short, HopM1 was found to trigger the degradation of AtMIN7 as part of its virulence function, leading to the hypothesis that P. syringae manipulates vesicle trafficking by targeted ARF-GEF (i.e., AtMIN7) degradation.
Dynamic responses to bacterial phytopathogen perception have also recently been shown to engage components of the actin cytoskeleton (Tian et al., 2009). Using a reverse genetic and biochemical approach, Tian and colleagues identified a regulator of stochastic actin dynamics (i.e., ACTIN DEPOLYMERIZING FACTOR-4; ADF4) as being required for the perception of P. syringae expressing the cysteine protease effector protein AvrPphB. In mutant Arabidopsis plants lacking ADF4, pathogen growth was unchecked, resulting in an increase in bacterial multiplication, leading to increased disease symptoms. This work further characterized the biochemical activity of the protein, and has led to the hypothesis that subtleties in depolymerization activity (i.e., actin binding, F-actin severing and depolymerization) may in fact account for some level of specificity regulating pathogen perception and the subsequent remodeling of the cortical actin cytoskeleton. Interestingly, this work also identified a link between actin depolymerization dynamics and the homeostatic control of hormone (i.e., SA and JA) physiology, further implicating the link between hormone signaling, host cell dynamics and the perception of pathogens by plants.
Transcriptional Regulation and Pathogenesis Related Genes
The common misconception is that plant defense responses are centrally regulated through protein-protein interactions While protein dynamics certainly account for a large proportion of the overall defense response (e.g., Mackey et al., 2002, 2003, Axtell and Staskawicz, 2003; Day et al., 2005, Liu et al., 2009; Lu et al., 2009), transcriptional regulation is a critical component in controlling plant resistance responses to pathogen infection (reviewed in Eulgem, 2005). Microarray analyses investigating the transcriptional reprogramming of defense signaling in Arabidopsis following inoculation with a variety of pathogens has revealed that in addition to the well established pathogenesis related (PR) genes (Sels et al., 2008), several hundred, even thousands, of genes undergo differential expression both during and following pathogen perception (Glazebrook, 2001). In fact, up to 25% of all Arabidopsis genes display altered transcript levels in response to pathogen infection (Maleck et al., 2000; Tao et al., 2003). Among these altered transcripts, members of several transcription factor families have also been implicated in defense gene regulation (Eulgem, 2005).
An early observation in response to pathogen infection is the expression of PR genes. PR genes are defined as the genes encoding for host proteins that accumulate after pathological or related stimuli (Van Loon and Van Strien, 1999). Currently, PR genes are classified into seventeen distinct families (Van Loon et al., 2006), including some of the most commonly used disease resistance markers PR1 and PDF1.2 (Ryals et al., 1996; Lay and Anderson, 2005). The PR-1 family of genes are some of the most ubiquitous, showing a strong conservation across species, and as such, appear to be represented across all plant species, with homologs present in fungi, insects and vertebrates (Van Loon et al., 2006). Despite being such a widely conserved group, relatively little is known of PR-1 family protein function in Arabidopsis disease resistance. Part of the difficulty in studying these genes are the number in Arabidopsis, with 22 PR-1-type genes present, as well as a widely-varied expression pattern; only a single member of the PR-1 gene family is activated by pathogen infection, insect feeding, or chemical treatment, while ten PR-1-type genes are constitutively expressed in roots and eight in pollen (Van Loon et al., 2006). In contrast to the PR-1 family, several additional PR groups have also been widely studied, including members of the PR-12 family, also known as defensins, which have members exhibiting antifungal activity. To this end, Terras et al. (1995) demonstrated in vitro antifungal activity to a wide range of fungi using purified PDF1.1. In a complementary series of experiments, Penninckx et al. (1996) showed in vitro antifungal activity to Alternaria brassicicola and Fusarium culmorum.
PR genes, in general, appear to be only a small portion of a larger defense-signaling network involving SA, JA and ethylene. Several compelling examples of this, discussed in Sels et al. (2008), include an analysis of disease resistance in ein2 mutants, defective in JA/ET signaling, as well as the SA signaling deficient npr1 mutant. The ein2 mutant was shown to have increased susceptibility to the necrotrophic fungal pathogen B. cinerea (Thomma et al., 1999), while showing decreased expression of several PR genes, including those from the PR-12, PR-3 and PR-4 gene families (Thomma et al., 2001). Likewise, the npr1 mutant showed increased susceptibility to many biotrophic pathogens including the bacterium P. syringae, with decreases in PR-1, PR-2 and PR-5 (Thomma et al., 2001).
a. Transcription Factors
In addition to the large, ubiquitous family of PR genes described above, representatives of the Arabidopsis TGA-bZIP, ERF, Myb, Whirly and WRKY families have been shown to bind defense related gene promoter elements and regulate their expression (reviewed in Eulgem, 2005). Binding sites of WRKY factors (W boxes) are ubiquitously conserved in upstream regions of genes up regulated during a variety of defense responses including SAR, R-protein-mediated disease resistance and basal defense (Maleck et al., 2000; Eulgem et al., 2004; Zipfel et al., 2004). Dong et al. (2003) showed the promoters of pathogen-inducible Arabidopsis WRKY genes were strongly enriched for W boxes, suggesting a role for feedback regulation by WRKYs themselves. Interestingly, the conservation of binding sites in defense genes is not limited to the WRKY family of transcription factors. The consensus binding motif of Whirly factors and a motif with similarity to ERF binding sites are conserved in promoters of genes expressed during incompatible interactions with Hyaloperonospora arabidopsidis (Eulgem et al., 2004).
The transcriptional cascade leading to the SA-dependent expression of PR1 is well established, and involves numerous transcription factors, such as WRKYs, NPR1 and TGAs (reviewed in Eulgem, 2005). For example, following activation of SA-responsive defense signaling, approximately 50 WRKY genes are “activated”, which in turn lead to the coordinate regulation of defense signaling; this represents both the accumulation and repression of differentially regulated transcript (Eulgem and Somssich, 2007). Among the best-characterized responses linking perception of SA and the activation of defense signaling is the activation of NPR1 transcription (Yu et al., 2001). Accumulation of SA also triggers a change in the redox status of NPR1, reducing it to a monomeric form that can then be translocated into the nucleus (Mou et al. 2003). Once inside the nucleus, NPR1 monomers are able to interact with members of the TGA-bZIP family of transcription factors (Fan and Dong, 2002), which in turn stimulates their binding to TGA boxes within the promoter of PR1 (reviewed in Singh et al., 2002). In total, this multi-step process leads to the activation and regulation of SA-dependent gene expression.
b. MAPK Signaling
Once pathogen perception has occurred, amplification and precise regulation of the signaling cascade is required. In both PTI and ETI, this amplification step typically involves the function of a suite of Mitogen-Activated Protein Kinases (MAPK) for downstream disease resistance signaling. The utility of MAPK signaling in plants is not restricted to biotic interactions; indeed, current literature is ripe with examples, including development, reproduction and response to environmental stress (reviewed in Andreasson and Ellis, 2010). Arabidopsis has 23 MAPKs, 10 MAPKKs and 60 MAPKKKs (hereafter collectively referred to as MAPKs; reviewed in Cvetkovskai et al., 2005). In total, the primary function of MAPKs is the transduction of signals originating from perception (i.e., ligand binding; e.g., FLS2-flg22 interaction) to the activation and regulation of a downstream target. Whether through protein-protein interactions, regulation of cellular trafficking or transcriptional activation, MAPKs have ubiquitous roles in the amplification and processing of stimuli from biotic and abiotic responses. For example, responses regulated by MAPKs that are specifically required for defense signaling include the hypersensitive response (HR), systemic acquired resistance (SAR), generation of reactive oxygen species (ROS) and the induction of PR gene expression. As detailed above, one of the primary downstream responses regulated by MAPK signaling is the transcriptional regulation of numerous genes associated with defense activation (reviewed in Eulgem, 2005).
THE HOST-PATHOGEN INTERFACE: LAYERED DEFENSES, RESISTANCE AND SUSCEPTIBILITY
Sequencing of the Arabidopsis genome opened the door to a plethora of resources enabling a detailed analysis of disease resistance signaling in plants. Homology-based analyses led to the identification of broadly conserved gene families, such as plant resistance genes (Aarts et al., 1998; Shen et al., 1998; Zhou et al., 2004). Coupled with forward and reverse genetic approaches, such as EMS mutagenesis and the subsequent functional characterization of candidate co-regulators (Century et al., 1995; Falk et al., 1999), signaling networks were soon assembled Through all of this, what is now evident is that disease resistance signaling in plants is a multi-layered network of perception, signal amplification and regulation. Crosstalk between these layers of responses mediates perception and specificity, as well as regulates the strength and duration of the response.
Elicitors and PAMP-Triggered Immunity
Plants have evolved the ability to recognize the somewhat basic features of a pathogen for the purpose of eliciting defense responses (reviewed in Zipfel, 2009). Among the earliest elicitors of a plant defense response to be characterized were the oligosaccharide polymers that constitute the outer cell walls of pathogenic organisms (Hahn et al., 1981). From a historical standpoint, PAMPs were first observed and characterized in early experiments by Anderson-Prouty and Albersheim (1975), which described the ability of a fungal cell wall component, b-glucan, to induce a defense response in plants. These experiments were followed by in-depth studies to identify additional PAMPs and their associated responses, including oligogalacturonides (Davis and Hahlbrock, 1987), chitin (Baureithel et al., 1994, Shibuya et al., 1996; Day et al., 2001; Okada et al., 2002; Miya et al., 2007; Wan et al., 2008) and chitosan (Hadwiger et al., 1981).
In 1999, Thomas Boller's group identified a single genetic locus in Arabidopsis that mediates the perception of what has become the best-characterized PAMP recognition response in plants: the FLS2-bacterial flagellin interaction (Gómez-Gómez et al., 1999). Looking back, the discovery of the flagellin receptor (i.e., FLS2; FLAGELLIN SENSITIVE-2) in Arabidopsis represents one of the seminal discoveries in molecular plant pathology. While the study of PAMP recognition in plants has a long history, until the identification of a specific PAMP receptor, the classical R-protein-effector interaction(s) was seen as the penultimate mechanism of disease resistance signaling, controlling specificity, host-range, recognition and the activation of immunity in plants (reviewed in Staskawicz et al., 2001). With FLS2, researchers were now confronting the possibility that plants coordinate parallel, and to a large extent, overlapping layers of defense signaling.
PTI occurs almost immediately following the physical interaction between host and pathogen (reviewed in Jones and Dangl, 2006). As noted above, the identification of FLS2 provided the first genetic evidence that PTI controls a broad range of both physiological and pathogen-specific resistance responses. In total, structure-function studies of the flg22-FLS2 interaction have contributed to the elucidation of signaling pathways and their associated mechanisms (Chinchilla et al., 2006; Robatzek et al., 2006; Göhre et al., 2008). However, what may be the greatest contribution of these studies is that they have provided an understanding of the spatial dynamics of signal perception and transduction (Robatzek et al., 2006; Heese et al., 2007).
FLS2 is a receptor protein kinase comprised of an extracellular leucine-rich repeat (LRR) domain and an intercellular cytoplasmic serine threonine kinase domain (Figure 2A; Gómez-Gómez and Boller, 2000). Following the perception of bacterial flagellin, FLS2 mediates the activation of broad-based plant defense responses, such as the activation of MAP kinase signaling (Asai et al., 2002), endosomal trafficking (Otegui and Spitzer, 2008) and regulation of stomata closure (Melotto et al., 2006) (Figure 2B). In short, binding of flagellin to FLS2 promotes the association with the receptor-like kinase BAK1 (Chinchilla et al., 2007; Heese et al., 2007), which is believed to trigger the activation of at least two MAP kinase cascades. In terms of regulating basal defense, genetic evidence seems to suggest that the MAP kinase kinases MKK1 and MKK2 negatively regulate immune responses in response to FLS2 activation (Ichimura et al., 2006; Qiu et al., 2008), while MPK3 and MPK6 are thought to positively regulate FLS2 immune responses (Bittel and Robatzek, 2007). To add to this complexity, evidence also points to the involvement of hormone signaling in regulating FLS2-mediated responses (Navarro et al., 2006; Tsuda et al., 2008).
As noted throughout this Chapter, the driving force behind the association of pathogens with plants is the acquisition of nutrients. In the first installment of The Arabidopsis Book chapter, which focused on the Arabidopsis-Pseudomonas interaction. Fumiaki Katagiri and colleagues pointed to the role of nutrient restriction as a basal defense response that promotes host disease resistance (Katagiri et al., 2002). After all, pathogens are not teleological beings; they are not attacking the plant, they are simply in search of nutrients, which in turn, provide a means to an end. As such, disease may simply be a consequence of a pathogen's search for nutrients, and as such, barriers, obstacles or processes that prevent the acquisition of nutrients must be circumvented or disabled. Pathogen entry may therefore be viewed as a “filter” that determines the success or demise of a pathogen. In support of this hypothesis, Melotto et al. (2006) demonstrated that the recognition of flg22 by FLS2 induces the rapid closure of stomata, thus restricting pathogen entry and subsequent proliferation. Interestingly, this restriction can be lifted through the action of a P. syringae-specific toxin, coronatine, which interferes with abscisic acid (ABA) signaling and stimulates stomata re-opening.
A common theme in the perception of pathogens by plants, as well as the subsequent signaling of defense-specific responses, is that there is a significant overlap in the regulation of general host physiology and the activation and regulation of defense responses. Whether through manipulating hormone balance, or through modulation of ubiquitous MAPK signaling pathways, plants have evolved broad mechanisms to sense and abrogate pathogen infection and proliferation. As such, the basal defense response may represent the most basic and ancient form of plant immunity (reviewed in Chisholm et al., 2006). As discussed in the following section, the evolution and adaptation of highly specific defense responses has occurred through gene-for-gene interactions.
ETI: Gene-for-Gene Resistance and the Guard Hypothesis
The current paradigm in host-pathogen interactions is that the activation of primary defense responses is initiated by PAMP recognition, which in turn leads to the activation of PTI (reviewed in Jones and Dangl, 2006). With the discovery of the first bacterial avirulence protein (Staskawicz et al., 1984), a new discipline in plant biology was born: molecular plant pathology. Approaches such as EMS mutagenesis, transposon tagging, as well as advances in gene expression and DNA sequencing made possible our ability to identify genetic elements responsible for the recognition of plant pathogens. As our understanding of how plants recognize and respond to pathogen infection increases, models have evolved, paradigms have shifted, and too, our approaches have adapted to advances in technology. The sequencing of the Arabidopsis genome made possible many of these advances, and more importantly, provided a pool of candidate defense components for further characterization. In this regard, it soon became evident that the classical gene-for-gene hypothesis could not fully explain the complex interactions between all plants and all pathogens.
In 1998, Van Der Biezen and Jones proposed what is now known as the “Guard Hypothesis” (Dangl and Jones, 2001) to explain the role of Prf in the AvrPto-Pto interaction, a model that has evolved to explain the complex surveillance mechanism(s) that controls host-pathogen interactions (Van Der Biezen and Jones, 1998). While this model does not fully explain all aspects of the dominant (e.g., R-protein-mediated) disease resistance responses in plants, it does provide a benchmark for investigating the genetic interactions between host R-proteins and their cognate pathogen effectors. Just a few years later, Dangl and Jones (2001) expressly laid out the foundations that we currently use to describe the Guard Hypothesis. In short, these include: R proteins may interact constitutively with their guardee; the effector and target may be associated in both susceptible and resistant hosts, with the R-protein as a member of the complex in a resistant host; and one host protein complex may be a target for multiple effectors. In 2002, Van der Hoorn et al. (2002) postulated three observations that they believed would generally validate the Guard model experimentally. First, when an R-protein serves as a guardee, there would be no direct interaction with the cognate effector protein. This was a critical step in addressing the few identified instances of direct R-effector interactions. Secondly, that the indirect interaction requires an additional host protein that is specific for each effector-R-protein pair. And finally, that this additional host protein's structure, or general occurrence, would qualify it as a candidate virulence target of the pathogen. As is often the case, the lack of evidence, or in this case, the inability to demonstrate direct interaction between effector-R-protein pairs has limited our ability to further explain the processes required for pathogen recognition. To this end, the Guard Hypothesis finally offered an answer to explain the interaction between host resistance proteins and cognate pathogen effectors. Perhaps more interesting at the time was the possibility that there existed an additional host protein that was unique for each interaction. However, one interesting caveat to this paradigm, as conceptualized in Dangl and Jones (2001), is the finding that an absolute uniqueness does not exist in all guard-guardee interactions (Mackey et al., 2002, 2003; Axtell et al., 2003; Day et al., 2005; Chisholm et al., 2006). To this end, an additional level of co-regulation exists among shared signaling networks comprising ETI.
a. Structure
Despite the ability of plants to recognize a wide range of pathogens, the suite of R-proteins present in most plants is somewhat limited in both structural and operational diversity. As shown in Figure 3A, R-proteins share a number of basic, common features, and in Arabidopsis, approximately 150 proteins comprise this family of disease resistance signaling mediators (Baumgarten et al., 2003). The largest class of R-genes encode for a nucleotide binding site-leucine-rich repeat (NB-LRR) class of proteins (Chisholm et al., 2006; Jones and Dangl, 2006). At the amino-terminus, the conserved nucleotide-binding (NB) site has been shown to be critical for ATP or GTP binding (Saraste et al., 1990). At the C-terminus, the LRR domain, which exhibits variability both in spatial organization (Istomin and Godzik, 2009) and length (Matsushima et al., 2009), is likely a platform for protein-protein interactions and peptide/ligand binding (Jones and Jones, 1996; Kajava, 1998). Not surprising, LRR domains are found in a diverse suite of proteins, ranging in function as regulators of processes controlling both development and plant defense (reviewed in Padmanabhan et al., 2009). The NB-LRR class of R-proteins can be further sub-divided based on N-terminal structural features (Chisholm et al., 2006). Among these, one type contains an N-terminal domain with homology to the Drosophila Toll and mammalian interleukin 1 receptors (TIR-NB-LRRs) while the other class contains putative coiled-coil domains (CC-NB-LRRs) (reviewed in Dangl and Jones, 2001). In Arabidopsis, the best-characterized R-proteins are members of the CC-NB-LRR proteins, such as RPM1 (RESISTANCE TO PSEUDOMONAS SYRINGAE PV. MACULICOLA-1; Bisgrove et al., 1994), RPS5 (RESISTANCE TO PSEUDOMONAS SYRINGAE-5; Simonich and Innes, 1995) and RPS2 (RESISTANCE TO PSEUDOMONAS SYRINGAE-2; Kunkel et al., 1993). Functionally, R-protein recognition of pathogen effectors, and/or the cellular perturbations elicited by the action of effectors, is critical to plant defense (Figure 3B). However, these actions alone (i.e., detection of perturbations) do not account for the full activation of disease resistance. To this end, additional plant proteins are required for proper R-protein function.
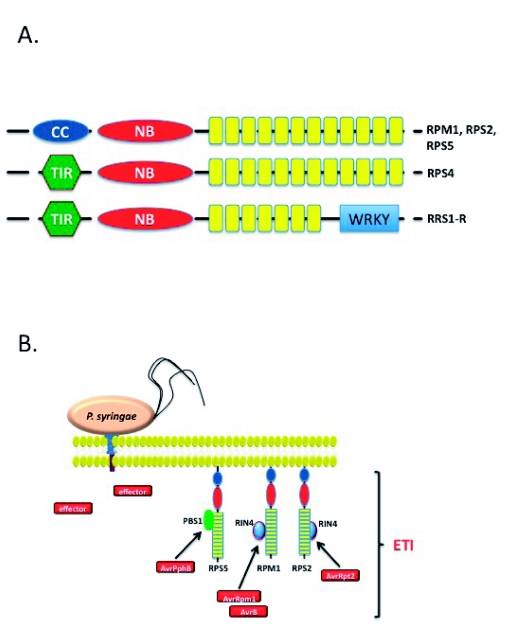
Figure 3:
Pathogen Effector Recognition and the Activation of Effector-Triggered Immunity.
A) The largest class of resistance proteins in Arabidopsis is the CC-NB-LRR class, whose members include the R-proteins RPM1, RPS2 and RPS5. The TIR-class of R-proteins are represented by the well-characterized R-protein RPS4. A recently identified variant of this class, RRS1-R, contains a WRKY domain believed to impart transcriptional regulation as part of its function following pathogen effector recognition.
B) Similar to the activation of PTI, the elicitation of ETI results in the activation of defense signaling via the specific recognition of pathogen-derived elicitors. As a second layer of defense signaling, ETI is the culmination in the recognition of pathogen effector proteins. As shown, delivery of an effector protein via the type III secretion system (T3SS) and subsequent recognition by cognate host R-proteins, leads to the activation of an amplified defense response. The general role of pathogen effector proteins is thought to be the inactivation of PTI, while the role of ETI is to block all mechanisms of pathogen virulence.
b. R-protein Stability and Activation
RAR1 (REQUIRED FOR MLA12 RESISTANCE-1) was demonstrated to be required for disease resistance mediated by several CC-NB-LRR, as well as at least one TIR-NB-LRR class R-protein (Muskett et al., 2002; Tornero et al., 2002). Evidence suggests that RAR1 may function through its physical interaction with another protein (i.e., SGT1, SUPPRESSOR OF G2 ALLELE OF SUPPRESSOR OF KINETOCHORE PROTEIN 1; Azevedo et al., 2002) that is also required for disease resistance mediated by several CC-NB-LRR and TIR-NB-LRRs (Azevedo et al., 2002). In short, the simplest model for a role of RAR1 in R-protein function is that it directs either the removal of a negative regulator (Gray et al., 1999) or the activation of a positive regulator (Wang et al., 2001) by recruitment of that factor to the SCF complex via SGT1 and subsequent ubiquitination (Tornero et al., 2002).
SGT1 was originally identified as a regulatory component of the Skp1, Cullin, F-box (SCF) complex (Bachmair et al., 2001) that acts as an E3 ligase involved in the ubiquitination of target proteins (Tornero et al., 2002). Since its identification, numerous genetic studies have implicated SGT1 as a key component in pathogen resistance signaling, most likely through regulating the expression levels and activities of R proteins (Peart et al., 2002). With the characterization of SGT1 interactions with the chaper-one HSP90, as well as with another protein, RAR1 (Takahashi et al., 2003), the shape of the complex regulatory node involving R-protein stability is starting to emerge (Figure 4). RAR1 is a member of the conserved CHORD-containing family (CHP, Shirasu et al., 1999), and is distinguished by the presence of two cysteine-and histidine-rich zinc-binding domains (CHORD I and CHORD II). In planta, RAR1 associates with SGT1, and this interaction appears to be required for full functionality of associated R-proteins (Austin et al., 2002; Azevedo et al., 2002; Peart et al., 2002; Tör et al., 2002). In mammals, Nod1 was recently shown to associate with HSP90, further confirming that studies first conducted in plants are invaluable about pathways involving NLR proteins in non-plant systems (Hahn, 2005). More recently, da Silva Correia (2007) also demonstrated that SGT1 is a positive regulator of Nod1 activation, providing compelling evidence that SGT1 is required for signaling by Nod1 in human cells, just as it is required in innate immune signaling in plants.
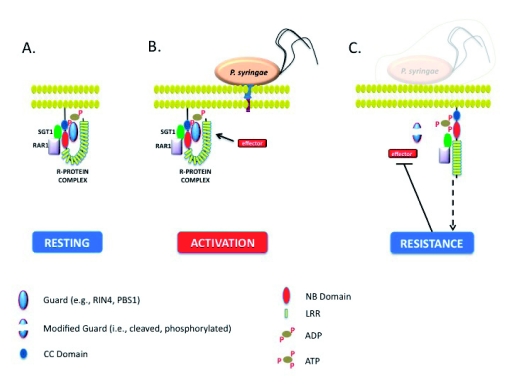
Figure 4:
A Model for the Regulation and Activation of R-Protein Mediated Defense Signaling in Arabidopsis.
A) In the absence of a pathogen, R-proteins are held in an inactive state. This conformation is the result of protein-proteins interaction(s), and as a consequence of these associations, binding of ATP/GTP to the NB domain is blocked.
B) Following perception of the pathogen, via the activity of secreted effector molecules, an induced conformational change in the R-protein complex is induced. This change results in a possible shift in the stoichiometry of protein-protein interactions, leading to the binding of ATP/GTP to the R-protein NB domain.
C) Once a pathogen effector is recognized, the activation of ETI results in the initiation of defense signaling, ultimately leading to the abrogation of pathogen growth. Once perturbations to the R-protein surveillance system are no longer perceived, the system resets back to the resting state depicted in “A”.
c. Regulators and Amplifiers of R-protein Signaling
In addition to the requirement for stabilizing and directing R-protein function, additional R-protein accessory proteins have been identified as being required for the activation of disease resistance signaling in plants. Among these, the best-characterized examples include ENHANCED DISEASE SUSCEPTIBILITY 1 (EDS1) and NON-RACE SPECIFIC DISEASE RESISTANCE 1 (NDR1). Both have been shown to be indispensible for the activation of disease resistance mediated by nearly all TIR-NB-LRRs and CC-NB-LRRs, respectively (Aarts et al., 1998). EDS1 has homology to eukaryotic lipases (Falk et al., 1999), and serves as a central regulatory protein in biotic and oxidative stress signaling (reviewed in Wiermer et al., 2005). Originally, EDS1 was identified in a screen for loss of resistance in Arabidopsis to isolates of the oomycete pathogen Hyaloperonospora arabidopsidis (Parker et al., 1996), and was the first plant L-family lipase representative to be cloned and assigned a function (Falk et al., 1999). In subsequent studies, eds1 mutants were also implicated in a loss of resistance to specific strains of P. syringae expressing effectors recognized by TIR-NB-LRRs such as RPP2, RPP4, RPP5, RPP21 and RPS4 (Aarts et al., 1998; Feys et al., 2001). In addition to the identification of a required role for EDS1 as a signaling protein in the TIR-NB-LRR network, significant advances were also made as a result of the identification of the first of two important interacting partners of EDS1, PHYTOALEXIN DEFICIENT 4 (PAD4), followed by the identification of an interaction with SENESCENCE-ASSOCIATED GENE 101 (SAG101) (Feys et al., 2005). PAD4 and SAG101 functions appear partially redundant, yet functionally independent. Feys et al. (2005) also demonstrated nuclear localization for EDS1 as well as EDS1:PAD4 and EDS1:SAG101 complexes, suggesting a dynamic role for EDS1 in defense signaling. Evidence also supports a role for EDS1, along with PAD4, in the plant response to oxidative stress (Rusterucci et al., 2001; Mateo et al., 2004), as well as being required for the runaway cell death response observed in LESION SIMULATING DISEASE 1 (LSD1) mutant plants caused by photooxidative stress (Mateo et al., 2004).
In addition to EDS1, a key regulator of CC-NB-LRR R-protein activation was also identified. A mutation in NDR1 was identified in a screen of fast-neutron mutagenized Col-0 Arabidopsis by screening for plants that became susceptible to P. syringae expressing the effector AvrB (discussed below; Century et al., 1995). The ndr1-1 mutant plant contains an approximately 1 kilo-base-pair deletion spanning the NDR1 locus on Arabidopsis chromosome three (Century et al., 1997). Functional characterization of NDR1 has revealed it to be a plasma membrane-localized protein of 219 amino acids (Century et al., 1997), which undergoes several post-translational modifications, including C-terminal processing and N-linked glycosylation (Coppinger et al., 2004). Interestingly, the proposed topology of NDR1 within the plasma membrane suggests that an approximate 18-amino acid portion lies within the cytoplasm, while the remainder of the NDR1 protein resides on the outside surface of the plasma membrane (Coppinger et al., 2004; Day et al., 2006). This hypothetical model raises the possibility that NDR1 positioning within the plasma membrane may serve to facilitate signaling from within the apoplast, across the plasma membrane, and into the cytoplasm, possibly through its interaction with RPM1 INTERACTING PROTEIN-4 (RIN4; Day et al., 2006). In total, NDR1 is required for the activation of many CC-NB-LRRs including RPS2, RPM1 and RPS5 (Century et al., 1995), and in support of this, ndr1-1 mutant plants are susceptible to P. syringae expressing the effector genes AvrB, AvrRpt2, AvrRpm1 and AvrPphB (Coppinger et al., 2004). As a required signaling component of multiple R-protein pathways in response to bacterial infection, NDR1 may also play a role in multiple disease resistance networks in plants. In support of this hypothesis, ndr1-1 plants show higher growth of P. syringae DC3000 (Century et al., 1995) suggesting the role of NDR1 may not be limited to only R-gene-mediated disease resistance, but could also be a critical component of PTI.
d. R-protein-Effector Interactions
In mammalian innate immune signaling, TLRs are responsible for the recognition of PAMPs, while their plant counterparts (i.e., R-proteins) are responsible for the recognition of secreted pathogen effector proteins As is the case in most receptor-ligand interactions, direct association between receptor and elicitor results in the stimulation and activation of down-stream signaling events required for activation. In total, this interaction results in the activation of signaling required for the successful deployment of defense responses and disease resistance. To date, two mechanisms of pathogen effector perception have been described in plants: direct and indirect recognition. In 2000, Jia et al (2000) demonstrated a direct interaction between the rice CC-NB-LRR R-protein Pita and its cognate effector protein AvrPita. This interaction specifies resistance in rice to the blast fungal pathogen Magnaporthe grisea. In short, this work demonstrated for the first time a direct interaction between a R-protein (receptor) and its ligand, a secreted effector from an invading pathogen. Subsequent work, as conceptually described above, identified this interaction as being mediated by the LRR domain of Pita (Bryan et al., 2000). Additional direct interactions have also been demonstrated between other R-proteins and their cognate pathogen effectors, such is the case with RRS1-R and the bacterial wilt pathogen effector PopP2 (Deslandes et al., 2003), as well as becoming the best-characterized examples from the flax rust resistance loci, which recognize approximately 30 effector proteins from flax rust (reviewed in Ellis et al., 2007). However, direct recognition of pathogen effector proteins appears to be the exception, rather than the rule.
As described above, the “rules” governing the proposition of the guard hypothesis satisfied the lack of additional direct interactions between host R-proteins and pathogen effectors. As an early example of the guard hypothesis, work in Roger Innes' lab demonstrated a multi-protein interaction that seemed to satisfy the criteria of an indirect surveillance mechanism (Simonich and Innes, 1995; Swiderski and Innes, 2001; Shao et al., 2003; Ade et al., 2007). In this case, the association of the Arabidopsis R-protein RPS5 with a protein kinase, PBS1, fulfilled all of the requirements of the Guard Hypothesis. This mechanism requires that: RPS5 associates/interacts with PBS1 (Ade et al., 2007); the P. syringae effector AvrPphB, a cysteine protease, cleaves PBS1 (Shao et al., 2003); and, following cleavage of PBS1, the RPS5-PBS1 association is disrupted, leading to a (likely) conformational change in RPS5 and activation of ETI (Ade et al., 2007). Thus, the detection of the pathogen relies on the disruption, or perturbation, of a protein-protein surveillance mechanism by the action of the pathogen effector protein. This, in short, defines ETI.
A separate series of experiments presented a new twist in the Guard Hypothesis; one that presented a testable model to explain the co-regulation and interplay between potentially overlapping defense signaling pathways. In 2002, the laboratory of Jeff Dangl presented the identification of a protein isolated as interacting with the CC-NB-LRR R-protein RPM1 (Mackey et al., 2002). This protein, RIN4, was shown to not only associate with RPM1, yet was also demonstrated to satisfy the requirement(s) as a guard of RPM1 activation. In short, the Pseudomonas effector protein AvrRPM1, which activates disease resistance through RPM1 (Bisgrove et al., 1994), was also demonstrated to act upon RIN4, most likely as an intermediate signaling component in this pathway (Mackey et al., 2002). The AvrRpm1-RIN4 interaction leads to the hyper-phosphorylation of RIN4 (Mackey et al., 2003) that is in turn recognized by RPM1. This interaction alone would seem to perfectly represent one of the original tenets of the Guard Hypothesis (Figure 3B). In a parallel series of experiments, RIN4 was also identified as a negative regulator of the RPS2-AvrRpt2 signaling pathway (Axtell and Staskawicz, 2003; Mackey et al., 2003). In this example, RIN4 is cleaved by AvrRpt2 (a cysteine protease), which in turn leads to the activation of ETI (Axtell and Staskawicz, 2003; Mackey et al., 2003; Day et al., 2005; Chisholm et al., 2006). While a host protein targeted by multiple effectors may not completely conform to the classical definition of the Guard Hypothesis, it provides an exceptional example of the overlapping regulation in parallel defense signaling networks. This demonstration of a shared intermediate (e.g., RIN4) has strengthened our understanding of the R-protein-effector interaction, and has paved the way for some of the newest concepts in molecular plant pathology. Additional recent studies have revealed a growing, almost ubiquitous, function for RIN4 in a variety of host-pathogen processes (Day et al., 2006; Liu et al., 2009; Luo et al., 2009; Wilton et al., 2010).
e. The Host-Pathogen Code
Another exciting discovery in the function of pathogen effectors is a recent series of experiments that are heralded as breaking the code of transcription activator-like (TAL) effector binding specificity. TAL family effectors are key virulence components of the Xanthomonas genus of bacterial plant pathogens (reviewed in Kay and Bonas, 2009). TAL effectors function by mimicking eukaryotic transcription factors (Gu et al, 2005; Schornack et al., 2006; Yang et al., 2006; Kay et al., 2007; Romer et al., 2007) and inducing gene expression leading to developmental changes contributing to disease symptoms (Kay and Bonas, 2009). In brief, TAL effectors are characterized by a central domain of tandem repeats, nuclear localization signals, and an acidic transcriptional activation domain (Van den Acerveken et al., 1996; Zhu et al., 1998; Schornack et al., 2006). The specific activity of a TAL effector is determined by the number and order of repeats (Herbers et al., 1992; Yang et al. 2005). Recently, Boch et al. (2009) identified the “code” used to designate sequence specificity in gene targets of the TAL effector AvrBs3. Furthermore, using this extrapolated “code”, Boch et al. (2009) were able to predict targets of other Xanthomonas TAL effectors. Perhaps most interesting, artificial TAL effectors were generated that could successfully target specified DNA sequence, suggesting the possibility that engineering specificity in plant-pathogen interactions is on the horizon.
FINAL THOUGHTS
So, which is it: Gene-for-Gene? The Guard Hypothesis? Are pathogen effector proteins the magic bullets that must be stopped? The more we learn, the more evident it becomes that we are only scratching the surface of what is to be understood in the field of plant-pathogen interactions. In the past few years, an explosion in the area of plant pathology has led to groundbreaking discoveries not only at the level of signaling and gene expression, but also in terms of the application of whole genome biology to non-model systems. In this regard, the early development and proposition that Arabidopsis is indeed a model system for translational agriculture is starting to become a reality. Moving forward in the next 10–20 years, a significant investment in the plant sciences will be to put into practice what has been learned from the studies highlighted above. Can we in fact tailor crops to recognize pathogens more efficiently, and too, can we engineer durable resistance to multiple pathogens across multiple crop species? Based on the overlapping specificity detailed above, one would imagine that through defining the shared mechanisms by which plants recognize diverse pathogens, the answer may be “YES!”. However, the complexity of disease resistance signaling, as well as the intimate links shared between disease resistance signaling and standard processes such as development, reproduction and, tolerance to additional environmental (i.e., abiotic) pressures, a balance must be struck in engineering disease resistance versus sacrificing plant health and vigor. Thus, in total, the end goal of molecular plant pathology is to understand the processes that ultimately make breeding for durable disease resistance a possibility. Whether this means that through understanding the function of all pathogen effectors we will be able to breed plants that are more resistant, or that by defining and better understanding the processes in plants that are minimally required for disease resistance, is still up for debate. Fortunately, there is much we still do not understand and a wealth of knowledge waiting to be discovered.
Acknowledgements
We would like to thank all members of the Day laboratory for critical reading of the manuscript. Research in the Day lab is supported by a grant from the National Science Foundation (IOS-0641319).
Footnotes
Citation: Knepper C., and Day B. (2010) From Perception to Activation: The Molecular-Genetic and Biochemical Landscape of Disease Resistance Signaling in Plants. The Arabidopsis Book 8:e0124. doi:10.1199/tab.0124
elocation-id: e0124
First published on May 14, 2010
References
- Aarts N., Metz M., Holub E., Staskawicz B.J., Daniels M.J., Parker J.E. Different requirements for EDS1 and NDR1 by disease resistance genes define at least two R gene-mediated signaling pathways in Arabidopsis. Proc. Natl. Acad. Sci. USA. 1998;958(1):10306–10311. doi: 10.1073/pnas.95.17.10306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ade J., DeYoung B.J., Golstein C., Innes R.W. Indirect activation of a plant nucleotide binding site-leucine-rich repeat protein by a bacterial protease. Proc. Natl. Acad. Sci. USA. 2007;1048(1):2531–2536. doi: 10.1073/pnas.0608779104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aist J.R.B., Bushnell W.R. In: The Fungal Spore and Disease Initiation in Plants and Animals. 1. Cole G. T., Hoch H. C., editors. Vol. 8. Plenum, New York: 1991. pp. 321–345. [Google Scholar]
- Aharoni A., Dixit S., Jetter R., Thoenes E., van Arkel G., Pereira A. The SHINE clade of AP2 domain transcription factors activates wax biosynthesis, alters cuticle properties, and confers drought tolerance when overexpressed in Arabidopsis. Plant Cell. 2004;168(1):2463–2480. doi: 10.1105/tpc.104.022897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad M., Cashmore A.R. HY4 gene of A. thaliana encodes a protein with characteristics of a blue-light photoreceptor. Nature. 1993;3668(1):110–111. doi: 10.1038/366162a0. [DOI] [PubMed] [Google Scholar]
- Anderson-Prouty A.J., Albersheim P. Host-Pathogen interactions: VIII. Isolation of a pathogen-synthesized fraction rich in glucan that elicits a defense response in the pathogen's host. Plant Physiol. 1975;568(1):286–291. doi: 10.1104/pp.56.2.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasson E., Ellis B. Convergence and specificity in the Arabidopsis MAPK nexus. Trends Plant Sci. 2010;158(1):106–113. doi: 10.1016/j.tplants.2009.12.001. [DOI] [PubMed] [Google Scholar]
- Asai T., Tena G., Plotnikova , Willmann M.R., Chiu W.L., Gomez-Gomez L., Boller T., Ausubel F.M., Sheen J. MAP kinase signaling cascade in Arabidopsis innate immunity. Nature. 2002;4158(1):977–983. doi: 10.1038/415977a. [DOI] [PubMed] [Google Scholar]
- Austin M.J., Muskett P., Kahn K., Feys B.J., Jones J.D., Parker J.E. Regulatory role of SGT1 in early R gene-mediated plant defenses. Science. 2002;2958(1):2077–2080. doi: 10.1126/science.1067747. [DOI] [PubMed] [Google Scholar]
- Axtell M.J., Staskawicz B.J. Initiation of RPS2-specified disease resistance in Arabidopsis is coupled to the AvrRpt2-directed elimination of RIN4. Cell. 2003;1128(1):369–377. doi: 10.1016/s0092-8674(03)00036-9. [DOI] [PubMed] [Google Scholar]
- Azevedo C., Sadanandom A., Kitagawa K., Freialdenhoven A., Shirasu K., Schulze-Lefert P. The RAR1 interactor SGT1, an essential component of R gene-triggered disease resistance. Science. 2002;2958(1):2073–2076. doi: 10.1126/science.1067554. [DOI] [PubMed] [Google Scholar]
- Bachmair A., Novatchkova M., Potuschak T., Eisenhaber F. Ubiquitination in plants: A post-genomic look at post-translational modification. Trends Plant Sci. 2001;68(1):463–470. doi: 10.1016/s1360-1385(01)02080-5. [DOI] [PubMed] [Google Scholar]
- Bari R., Jones J.D. Role of plant hormones in plant defence responses. Plant Mol. Biol. 2009;698(1):473–488. doi: 10.1007/s11103-008-9435-0. [DOI] [PubMed] [Google Scholar]
- Baumgarten A., Cannon S., Spangler R., May G. Genome-level evolution of resistance genes in Arabidopsis thaliana. Genetics. 2003;1658(1):309–319. doi: 10.1093/genetics/165.1.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baureithel K., Felix G., Boller T. Specific, high affinity binding of chitin fragments to tomato cells and membranes. Competitive inhibition of binding by derivatives of chitooligosaccharides and Nod factor of Rhizobium. J. Biol. Chem. 1994;2698(1):17931–17938. [PubMed] [Google Scholar]
- Bisgrove S.R., Simonich S.T., Smith N.M., Sattler A., Innes R.W. A disease resistance gene in Arabidopsis with specificity for two different pathogen avirulence genes. Plant Cell. 1994;68(1):927–933. doi: 10.1105/tpc.6.7.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittel P., Robatzek S. Microbe-associated molecular patterns (MAMPs) probe immunity. Curr Opin. Plant Biol. 2007;108(1):335–341. doi: 10.1016/j.pbi.2007.04.021. [DOI] [PubMed] [Google Scholar]
- Block A., Guo M., Elowsky C., Clemente T.E., Alfano J.R. The Pseudomonas syringae type III effector HopG1 targets mitochondria, alters plant development and suppresses plant innate immunity. Cell Microbiol. 2010;128(1):318–330. doi: 10.1111/j.1462-5822.2009.01396.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boch J., Scholze H., Schornack S., Landgraf A., Hahn S., Kay S., Lahaye T., Nickstadt A., Bonas U. Breaking the Code of DNA Binding Specificity of TAL-Type III Effectors. Science. 2009;3268(1):1509–1512. doi: 10.1126/science.1178811. [DOI] [PubMed] [Google Scholar]
- Browne L.M., Conn K.L., Ayer W.A., Tewari J.P. The camalexins: new phytoalexins produced in the leaves of Camelina sativa (Cruciferae). Tetrahedron. 1991;478(1):3909–3914. [Google Scholar]
- Bryan G.T., Wu K.S., Farrall L., Jia Y., Hershey H.P., McAdams S.A., Faulk K.N., Donaldson G.K., Tarchini R., Valent B. A single amino acid difference distinguishes resistant and susceptible alleles of the rice blast resistance gene Pi-ta. Plant Cell. 2000;128(1):2033–2046. doi: 10.1105/tpc.12.11.2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Century K., Holub E.B., Staskawicz B.J. NDR1, a locus of Arabidopsis thaliana that is required for disease resistance to both a bacterial and fungal pathogen. Proc. Natl. Acad. Sci. USA. 1995;928(1):6597–6601. doi: 10.1073/pnas.92.14.6597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Century K., Shapiro A.D., Repetti P.P., Dahlbeck D., Holub E., Stascawicz B.J. NDR1, a pathogen-induced component required for Arabidopsis disease resistance. Science. 1997;2788(1):1963–1965. doi: 10.1126/science.278.5345.1963. [DOI] [PubMed] [Google Scholar]
- Chandra A., Huff D.R. A fungal parasite regulates a putative female-suppressor gene homologous to maize tasselseed2 and causes induced hermaphroditism in male buffalograss. Mol. Plant Microbe Interact. 2010;238(1):239–250. doi: 10.1094/MPMI-23-3-0239. [DOI] [PubMed] [Google Scholar]
- Chen Y.F., Shakeel S.N., Bowers J., Zhao X.C., Etheridge N., Schaller G.E. Ligand-induced degradation of the ethylene receptor ETR2 through a proteasome-dependent pathway in Arabidopsis. 2007;2828(1):24752–24758. doi: 10.1074/jbc.M704419200. [DOI] [PubMed] [Google Scholar]
- Chinchilla D., Bauer Z., Regenass M., Boller T., Felix G. The Arabidopsis receptor kinase FLS2 binds flg22 and determines the specificity of flagellin perception. 2006;188(1):465–476. doi: 10.1105/tpc.105.036574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinchilla D., Zipfel C., Robatzek S., Kemmerling B., Nurnberger T., Jones J.D., Felix G., Boller T. A flagellin-induced complex of the receptor FLS2 and BAK1 initiates plant defence. Nature. 2007;4488(1):497–500. doi: 10.1038/nature05999. [DOI] [PubMed] [Google Scholar]
- Chisholm S.T., Coaker G., Day B., Staskawicz B.J. Host-Microbe Interactions: Shaping the Evolution of the Plant Immune Response. Cell. 2006;1248(1):803–814. doi: 10.1016/j.cell.2006.02.008. [DOI] [PubMed] [Google Scholar]
- Clay N.K., Adio A.M., Denoux C., Jander G., Ausubel F.M. Glucosinolate metabolites required for Arabidopsis innate immune response. Science. 2009;3238(1):95–101. doi: 10.1126/science.1164627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins N.C., Thordal-Christensen H., Lipka V., Bau S., Kombrink E., Qiu J.L., Huckelhoven R., Stein M., Freialdenhoven A., Somerville S.C., Schulze-Lefert P. SNARE-protein-mediated disease resistance at the plant cell wall. Nature. 2003;4258(1):973–977. doi: 10.1038/nature02076. [DOI] [PubMed] [Google Scholar]
- Coppinger P., Repetti P.P., Day B., Dahlbeck D., Mehlert A., Staskawicz B.J. Overexpression of the plasma membrane-localized NDR1 protein results in enhanced bacterial disease resistance in Arabidopsis thaliana. Plant J. 2004;408(1):225–237. doi: 10.1111/j.1365-313X.2004.02203.x. [DOI] [PubMed] [Google Scholar]
- Cui J., Bahrami A.K., Pringle E.G., Hernandez-Guzman G., Bender C.L., Pierce N.E., Ausubel F.M. Pseudomonas syringae manipulates systemic plant defenses against pathogens and herbivores. Proc. Natl. Acad. Sci. USA. 2005;1028(1):1791–1796. doi: 10.1073/pnas.0409450102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva Correia J., Miranda Y., Leonard N., Ulevitch R. SGT1 is essential for Nod1 activation. Proc. Natl. Acad. Sci. USA. 2007;1048(1):6764–6769. doi: 10.1073/pnas.0610926104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dangl J.L., Jones J.D.G. Plant pathogens and integrated defence responses to infection. Nature. 2001;4118(1):826–833. doi: 10.1038/35081161. [DOI] [PubMed] [Google Scholar]
- Davis K.R, Hahlbrock K. Induction of Defense Responses in Cultured Parsley Cells by Plant Cell Wall Fragments. Plant Physiol. 1987;848(1):1286–1290. doi: 10.1104/pp.84.4.1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day B., Okada M., Ito Y., Tsukada K., Zaghouani H., Shibuya N., Stacey G. Binding site for chitin oligosaccharides in the soy-bean plasma membrane. Plant Physiol. 2001;1268(1):1162–1173. doi: 10.1104/pp.126.3.1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day B., Dahlbeck D., Huang J., Chisholm S.T., Li D., Staskawicz B.J. Molecular basis for the RIN4 negative regulation of RPS2 disease resistance. Plant Cell. 2005;178(1):1292–1305. doi: 10.1105/tpc.104.030163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day B., Dahlbeck D., Staskawicz B.J. NDR1 interaction with RIN4 mediates the differential activation of multiple disease resistance pathways in Arabidopsis. Plant Cell. 2006;188(1):2782–2791. doi: 10.1105/tpc.106.044693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Wit P.J., Mehrabi R., Van den Burg H.A., Steriopoulos I. Fungal effector proteins; past, present and future. Mol. Plant Pathol. 2009;108(1):735–747. doi: 10.1111/j.1364-3703.2009.00591.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denoux C., Galletti R., Mammarella N., Gopalana S., Werck D., De-Lorenzo G., Ferrari S., Ausubel F.M., Dewdney J. Activation of defense response pathways by OGs and Flg22 elicitors in Arabidopsis seedlings. Mol. Plant. 2008;18(1):423–445. doi: 10.1093/mp/ssn019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deslandes L., Olivier J., Peeters N., Feng D.X., Khounlotham M., Boucher C., Somssich I., Genin S., Marco Y. Physical interaction between RRS-R, a protein conferring resistance to bacterial wilt, and PopP2, a type III effector targeted to the plant nucleus. Proc Natl. Acad. Sci USA. 2003;1008(1):8024–8029. doi: 10.1073/pnas.1230660100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong J, Chen C., Chen Z. Expression profiles of the Arabidopsis WRKY gene superfamily during plant defense response. Plant Mol. Biol. 2003;518(1):21–37. doi: 10.1023/a:1020780022549. [DOI] [PubMed] [Google Scholar]
- Ellis J.G., Dodds P.N., Lawrence G.J. The role of secreted proteins in diseases of plants caused by rust, powdery mildew, and smut fungi. Curr. Opin. Microbiol. 2007;108(1):326–331. doi: 10.1016/j.mib.2007.05.015. [DOI] [PubMed] [Google Scholar]
- Eulgem T. Regulation of the Arabidopsis defense transcriptome. Trends Plant Sci. 2005;108(1):71–78. doi: 10.1016/j.tplants.2004.12.006. [DOI] [PubMed] [Google Scholar]
- Eulgem T., Weigman V.J., Chang H.S., McDowell J.M., Holub E.B., Glazebrook J., Zhu T., Dangl J.L. Gene expression signatures from three genetically separable resistance gene signaling pathways for downy mildew resistance. Plant Physiol. 2004;1358(1):1129–1144. doi: 10.1104/pp.104.040444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eulgem T., Somssich I.E. Networks of WRKY transcription factors in defense signaling. Curr. Opin. Plant Biol. 2007;108(1):366–371. doi: 10.1016/j.pbi.2007.04.020. [DOI] [PubMed] [Google Scholar]
- Falk A., Feys B.J., Frost L.N., Jones J.D.G., Daniels M.J., Parker J.E. EDS1, an essential component of R gene-mediated disease resistance in Arabidopsis has homology to eukaryotic lipases. Proc. Natl. Acad. Sci. USA. 1999;968(1):3292–3297. doi: 10.1073/pnas.96.6.3292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan W., Dong X. In vivo interaction between NPR1 and transcription factor TGA2 leads to salicylic acid-mediated gene activation in Arabidopsis. Plant Cell. 2002;148(1):1377–1389. doi: 10.1105/tpc.001628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feys B.J., Moisan L.J., Newman M.A., Parker J.E. Direct interaction between the Arabidopsis disease resistance signaling proteins, EDS1 and PAD4. EMBO J. 2001;208(1):5400–5411. doi: 10.1093/emboj/20.19.5400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feys B.J., Wiermer M., Bhat R.A., Moisan L.J., Medina-Escobar N., Neu C., Cabral A., Parker J.E. Arabidopsis SENES-CENCE-ASSOCIATED GENE101 stabilizes and signals within an ENHANCED DISEASE SUSCEPTIBILITY1 complex in plant innate immunity. Plant Cell. 2005;178(1):2601–2613. doi: 10.1105/tpc.105.033910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glawischnig E. Camalexin. Phytochemistry. 2007;688(1):401–406. doi: 10.1016/j.phytochem.2006.12.005. [DOI] [PubMed] [Google Scholar]
- Glazebrook J. Genes controlling expression of defense responses in Arabidopsis-2001 status. Curr. Opin. Plant Biol. 2001;48(1):301–308. doi: 10.1016/s1369-5266(00)00177-1. [DOI] [PubMed] [Google Scholar]
- Göhre V., Spallek T., Haweker H., Mersmann S., Mentzel T., Boller T., de Torres M., Mansfield J.W., Robatzek S. Plant pattern-recognition receptor FLS2 is directed for degradation by the bacterial ubiquitin ligase AvrPtoB. Curr. Biol. 2008;188(1):1824–1832. doi: 10.1016/j.cub.2008.10.063. [DOI] [PubMed] [Google Scholar]
- Gómez-Gómez L, Felix G, Boller T. A single locus determines sensitivity to bacterial flagellin in Arabidopsis thaliana. Plant J. 1999;188(1):277–284. doi: 10.1046/j.1365-313x.1999.00451.x. [DOI] [PubMed] [Google Scholar]
- Gómez-Gómez L., Boller T. FLS2: an LRR receptor-like kinase involved in the perception of the bacterial elicitor flagellin in Arabidopsis. Mol. Cell. 2000;58(1):1003–1011. doi: 10.1016/s1097-2765(00)80265-8. [DOI] [PubMed] [Google Scholar]
- Gray W.M., del Pozo J.C., Walker L., Hobbie L., Risseeuw E., Banks T., Crosby W.L., Yang M., Ma H., Estelle M. Identification of an SCF ubiquitin-ligase complex required for auxin response in Arabidopsis thaliana. Genes Dev. 1999;138(1):1678–1691. doi: 10.1101/gad.13.13.1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu K., Yang B., Tian D., Wu L., Wang D., Sreekala C., Yang F., Chu Z., Wang G.L., White F.F., Yin Z. R gene expression induced by a type-III effector triggers disease resistance in rice. Nature. 2005;4358(1):1122–1125. doi: 10.1038/nature03630. [DOI] [PubMed] [Google Scholar]
- Hadwiger L.A., Beckman J.M., Adams M.J. Localization of fungal components in the pea-Fusarium interaction detected immunochemically with anti-chitosan and anti-fungal cell wall antisera. Plant. Physiol. 1981;678(1):170–175. doi: 10.1104/pp.67.1.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn H., Kaufmann S.H.E. The role of cell-mediated immunity in bacterial infections. Rev. Infect. Dis. 1981;38(1):1221–1250. doi: 10.1093/clinids/3.6.1221. [DOI] [PubMed] [Google Scholar]
- Hahn J.S. Regulation of Nod1 by Hsp90 chaperone complex. FEBS Lett. 2005;579 doi: 10.1016/j.febslet.2005.07.024. [DOI] [PubMed] [Google Scholar]
- Hamilton A.J., Baulcombe D.C. A species of small antisense RNA in posttranscriptional gene silencing in plants. Science. 1999;2868(1):950–952. doi: 10.1126/science.286.5441.950. [DOI] [PubMed] [Google Scholar]
- Hematy K., Cherk C., Somerville S. Host-pathogen warfare at the plant cell wall. Curr. Opin. Plant Biol. 2009;128(1):406–413. doi: 10.1016/j.pbi.2009.06.007. [DOI] [PubMed] [Google Scholar]
- Heese A., Hann D.R., Gimenez-Ibanez S., Jones A.M., He K., Li J., Schroeder J.I., Peck S.C., Rathjen J.P. The receptor-like kinase SERK3/BAK1 is a central regulator of innate immunity in plants. Proc. Natl. Acad. Sci. USA. 2007;1048(1):12217–12222. doi: 10.1073/pnas.0705306104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbers K., Conrads-Strauch J., Bonas U. Race-specifi city of plant resistance to bacterial spot disease determined by repetitive motifs in a bacterial avirulence protein. Nature. 1992;3568(1):172–174. [Google Scholar]
- Hirano S.S., Upper C.D. Bacteria in the leaf ecosystem with emphasis on Pseudomonas syringae-a pathogen, ice nucleus, and epiphyte. Microbiol. Mol. Biol. Rev. 2000;648(1):624–653. doi: 10.1128/mmbr.64.3.624-653.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichimura K., Casais C., Peck S.C., Shinozaki K., Shirasu K. MEKK! Is required for MPK4 activation and regulates tissuespecific and temperature-dependent cell death in Arabidopsis. J. Biol. Chem. 2006;2818(1):36969–36976. doi: 10.1074/jbc.M605319200. [DOI] [PubMed] [Google Scholar]
- Irani N.G., Russinova E. Receptor endocytosis and signaling in plants. Curr. Opin. Plant Biol. 2009;128(1):653–659. doi: 10.1016/j.pbi.2009.09.011. [DOI] [PubMed] [Google Scholar]
- Istomin A.Y., Godzik A. Understanding diversity of human innate immunity receptors: analysis of surface features of leucine-rich repeat domains in NLRs and TLRs. BMC Immunol. 2009;38(1):1–15. doi: 10.1186/1471-2172-10-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffree C.E. The fine structure of the plant cuticle. Biology of the plant cuticle. In: Reiderer M., Muller C., editors. Annual Plant Reviews. 1. Vol. 23. Vol. 8. Blackwell publishing Ltd; 2006. pp. 11–16. [Google Scholar]
- Jia Y., McAdams S.A., Bryan G.T., Hershey H.P., Valent B. Direct interaction of resistance gene and avirulence gene products confers rice blast resistance. EMBO J. 2000;198(1):4004–4014. doi: 10.1093/emboj/19.15.4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones A.M., Chory J., Dangl J.L., Estelle M., Jacobsen S.E., Meyerowitz E.M., Nordborg M., Weigel D. The Impact of Arabidopsis on Human Health: Diversifying Our Portfolio. Cell. 2008;1338(1):939–943. doi: 10.1016/j.cell.2008.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones D.A., Jones J.D.G. The roles of leucine rich repeats in plant defences. Adv. Bot Res. Adv. Plant Pathol. 1996;248(1):90–167. [Google Scholar]
- Jones J.D., Dangl J.L. The plant immune system. Nature. 2006;4448(1):323–329. doi: 10.1038/nature05286. [DOI] [PubMed] [Google Scholar]
- Kajava A.V. Structural diversity of leucine-rich repeat proteins. J. Mol. Biol. 1998;2778(1):519–527. doi: 10.1006/jmbi.1998.1643. [DOI] [PubMed] [Google Scholar]
- Katagiri F., Thilmony R., He S.Y. The Arabidopsis Thaliana-Pseudomonas Syringae Interaction. In: Last R., editor. The Arabidopsis Book. American Society of Plant Biologists; 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay S., Bonas U. How Xanthomonas type III effectors manipulate the host plant. Curr. Opin. Microbiol. 2009;128(1):37–43. doi: 10.1016/j.mib.2008.12.006. [DOI] [PubMed] [Google Scholar]
- Kay S., Hahn S., Marois E., Hause G., Bonas U. A bacterial effector acts as a plant transcription factor and induces a cell size regulator. Science. 2007;3188(1):648–651. doi: 10.1126/science.1144956. [DOI] [PubMed] [Google Scholar]
- Kim C.Y., Bove J., Assmann S.M. Overexpression of wound-responsive RNA-binding proteins induces leaf senescence and hypersensitive-like cell death. New Phytol. 2008;1808(1):57–70. doi: 10.1111/j.1469-8137.2008.02557.x. [DOI] [PubMed] [Google Scholar]
- Kliebenstein D.J., Rowe H.C., Denby K.J. Secondary metabolites influence Arabidopsis/Botrytis interactions: variation in host production and pathogen sensitivity. Plant J. 2005;448(1):25–36. doi: 10.1111/j.1365-313X.2005.02508.x. [DOI] [PubMed] [Google Scholar]
- Lay F.T., Anderson M.A. Defensins-components of the innate immune system in plants. Curr. Protein Pept. Sci. 2005;68(1):85–101. doi: 10.2174/1389203053027575. [DOI] [PubMed] [Google Scholar]
- Lindeberg M., Cunnac S., Collmer A. The evolution of Pseudomonas syringae host specificity and type III effector repertoires. Mol. Plant Pathol. 2009;108(1):767–775. doi: 10.1111/j.1364-3703.2009.00587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipka V., Dittgen J., Bednarek P., Bhat R., Wiermer M., Stein M., Landtag J., Brandt W., Rosahl S., Scheel D., Llorente F., Molina A., Parker J., Somerville S., Schulze-Lefert P. Pre- and post-invasion defenses both contribute to nonhost resistance in Arabidopsis. Science. 2005;3108(1):1180–1183. doi: 10.1126/science.1119409. [DOI] [PubMed] [Google Scholar]
- Lipka U., Fuchs R., Lipka V. Arabidopsis non-host resistance to powdery mildews. Curr. Opin. Plant Biol. 2008;118(1):404–411. doi: 10.1016/j.pbi.2008.04.004. [DOI] [PubMed] [Google Scholar]
- Liu J., Elmore J.M., Fuglsang A.T., Palmgren M.G., Staskawicz B.J., Coaker G. RIN4 functions with plasma membrane H+-ATPases to regulate stomatal aperatures during pathogen attack. PLoS Biol. 2009;78(1):e1000139. doi: 10.1371/journal.pbio.1000139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X., Tintor N., Mentzel T., Kombrink E., Boller T., Robatzek S., Schulze-Lefert P., Saijo Y. Uncoupling of sustained MAMP receptor signaling from early outputs in an Arabidopsis endoplasmic reticulum glucosidase II allele. Proc. Natl. Acad. Sci. USA. 2009;1068(1):22522–22527. doi: 10.1073/pnas.0907711106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y., Caldwell K.S., Wroblewski T., Wright M.E., Michelmore R.W. Proteolysis of a negative regulator of innate immunity is dependent on resistance genes in tomato and Nicotiana benthamiana and induced by multiple bacterial effectors. Plant Cell. 2009;218(1):2458–2472. doi: 10.1105/tpc.107.056044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackey D., Holt B.F., Wiig A., Dangl J.L. RIN4 interacts with Pseudomonas syringae type III effector molecules and is required for RPM1-mediated resistance in Arabidopsis. Cell. 2002;1088(1):743–754. doi: 10.1016/s0092-8674(02)00661-x. [DOI] [PubMed] [Google Scholar]
- Mackey D., Belkhadir Y., Alonso J.M., Ecker J.R., Dangl J.L. Arabidopsis RIN4 is the target of the type III virulence effector AvrRpt2 and modulates RPS2 mediated resistance. Cell. 2003;1128(1):379–389. doi: 10.1016/s0092-8674(03)00040-0. [DOI] [PubMed] [Google Scholar]
- Maleck K., Levine A., Eulgem T., Morgan A., Schmid J., Lawton K.A., Dangl J.L., Dietrich R.A. The transcriptome of Arabidopsis thaliana during systemic acquired resistance. Nat. Genet. 2000;268(1):403–410. doi: 10.1038/82521. [DOI] [PubMed] [Google Scholar]
- Mateo A., Muhlenbock P., Rusterucci C., Chang C.C., Miszalski Z., Karpinska B., Parker J.E., Mullineaux P.M., Karpinski S. LESION SIMULATING DISEASE 1 is required for acclimation to conditions that promote excess excitation energy. Plant Physiol. 2004;1368(1):2818–2830. doi: 10.1104/pp.104.043646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushima N., Mikami T., Tanaka T., Miyashita H., Yamada K., Kuroki Y. Analyses of non-leucine-rich repeat (non-LRR) regions intervening between LRRs in proteins. Biochim Biophys Acta. 2009;17908(1):1217–1237. doi: 10.1016/j.bbagen.2009.06.014. [DOI] [PubMed] [Google Scholar]
- Matzinger P. Friendly and dangerous signals: is the tissue in control? Nat. Immunol. 2007;88(1):11–13. doi: 10.1038/ni0107-11. [DOI] [PubMed] [Google Scholar]
- Melotto M., Underwood W., Koczan J., Nomura K., He S.Y. Plant stomata function innate immunity against bacterial invasion. Cell. 2006;1268(1):969–980. doi: 10.1016/j.cell.2006.06.054. [DOI] [PubMed] [Google Scholar]
- Miya A., Albert P., Shinya T., Desaki Y., Ichimura K., Shirasu K, Narusaka Y., Kawakami N., Kaku H., Shibuya N. CERK1, a LysM receptor kinase, is essential for chitin elicitor signaling in Arabidopsis. Proc. Natl. Acad. Sci. USA. 2007;1048(1):19613–19618. doi: 10.1073/pnas.0705147104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan W., Kamoun S. RXLR effectors of plant pathogenic oomycetes. Curr. Opin. Microbiol. 2007;108(1):332–338. doi: 10.1016/j.mib.2007.04.005. [DOI] [PubMed] [Google Scholar]
- Mou Z., Fan W., Dong X. Inducers of plant systemic acquired resistance regulate NPR1 function through redox changes. Cell. 2003;1138(1):935–944. doi: 10.1016/s0092-8674(03)00429-x. [DOI] [PubMed] [Google Scholar]
- Muskett P.R., Kahn K, Austin M.J., Moisan L.J., Sadanandom A., Shirasu K., Jones J.D.G., Parker J.E. Arabidopsis PAR1 exerts rate-limiting control of R gene mediated defenses against multiple pathogens. Plant Cell. 2002;148(1):979–992. doi: 10.1105/tpc.001040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mysore K.S., Ryu C. Nonhost resistance: how much do we know? Trends Plant Sci. 2004;98(1):97–104. doi: 10.1016/j.tplants.2003.12.005. [DOI] [PubMed] [Google Scholar]
- Navarro L., Dunoyer P., Jay F., Arnold B., Dharmasiri N., Estelle M., Voinnet O., Jones J.D. A plant miRNA contributes to antibacterial resistance by repressing auxin signaling. Science. 2006;3128(1):436–439. doi: 10.1126/science.1126088. [DOI] [PubMed] [Google Scholar]
- Nawrath C. Unraveling the complex network of cuticular structure and function. Curr. Opin. Plant Biol. 2006;98(1):281–287. doi: 10.1016/j.pbi.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Nomura K., Debroy S., Lee Y.H., Pumplin N., Jones J., He S.Y. A bacterial virulence protein suppresses host innate immunity to cause plant disease. Science. 2006;3138(1):220–223. doi: 10.1126/science.1129523. [DOI] [PubMed] [Google Scholar]
- Okada M., Matsumura M., Ito Y., Shibuya N. High-affinity binding proteins for N-Acetylchitooligosaccharide elicitor in the plasma membranes from wheat, barley, and carrot cells: conserved presence and correlation with the responsiveness to the elicitor. Plant Cell Physiol. 2002;438(1):505–512. doi: 10.1093/pcp/pcf060. [DOI] [PubMed] [Google Scholar]
- Otegui M.S., Spitzer C. Endosomal functions in plants. Traffic. 2008;98(1):1589–1598. doi: 10.1111/j.1600-0854.2008.00787.x. [DOI] [PubMed] [Google Scholar]
- Padmanabhan M., Cournoyer P., Dinesh-Kumar S.P. The leucine-rich repeat domain in plant innate immunity: a wealth of possibilities. Cell Microbiol. 2009;118(1):191–198. doi: 10.1111/j.1462-5822.2008.01260.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker J.E., Holub E.B., Frost L.N., Falk A., Gunn N.D., Daniels M.J. Characterization of eds1, a mutation in Arabidopsis suppressing resistance to Peronospora parasitica specified by several different RPP genes. Plant Cell. 1996;88(1):2033–2046. doi: 10.1105/tpc.8.11.2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peart J.R., Lu R., Sadanandom A., Malcuit I., Moffett P., Brice D.C., Schauser L., Jaggard D.A.W., Xiao S., Coleman M.J., Dow M., Jones J.D.G., Shirasu , Baulcombe D.C. Ubiquitin ligase-associated protein SGT1 is required for host and nonhost disease resistance in plants. Proc. Natl. Acad. Sci. USA. 2002;998(1):10865–10869. doi: 10.1073/pnas.152330599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedley K.F., Martin G.B. Molecular basis of Pto-mediated resistance to bacterial speck disease in tomato. Annu. Rev. Phytopathol. 2003;418(1):215–243. doi: 10.1146/annurev.phyto.41.121602.143032. [DOI] [PubMed] [Google Scholar]
- Pedras M.S., Khan A.Q. Unprecedented detoxification of the cruciferous phytoalexin camalexin by a root phytopathogen. Bioorg. Med. Chem. Lett. 1997;78(1):2255–2260. [Google Scholar]
- Pedras M.S., Khan A.Q. Biotransformation of the phytoalexin camalexin by the phytopathogen Rhizoctonia solani. Phytochemistry. 2000;538(1):59–69. doi: 10.1016/s0031-9422(99)00479-3. [DOI] [PubMed] [Google Scholar]
- Penninckx I.A., Eggermont K., Terras F.R., Thomma B.P., De Samblanx G.W., Buchala A., Metraux J.P., Manners J.M., Broekaert W.F. Pathogen-induced systemic activation of a plant defensin gene in Arabidopsis follows a salicylic acid-independent pathway. Plant Cell. 1996;88(1):2309–2323. doi: 10.1105/tpc.8.12.2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu J., Zhou L., Yun B., Nielsen H.B., Fiil B.K., Petersen K., MacKinlay J., Loake G. J., Mundy J., Morris P.C. Arabidopsis mitogen-activated protein kinases MKK1 and MKK2 have overlapping functions in defense signaling mediated by MEKK1, MPK4, and MKS1. Plant Physiol. 2008;1488(1):212–222. doi: 10.1104/pp.108.120006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reina-Pinto J. J., Yephremov A. Surface lipids and plant defenses. Plant Physiol. Biochem. 2009;478(1):540–549. doi: 10.1016/j.plaphy.2009.01.004. [DOI] [PubMed] [Google Scholar]
- Robatzek S., Chinchilla D., Boller T. Ligand-induced endocytosis of the pattern recognition receptor FLS2 in Arabidopsis. Genes Dev. 2006;208(1):537–542. doi: 10.1101/gad.366506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Römer P., Hahn S., Jordan T., Strauss T., Bonas U., Lahaye T. Plant pathogen recognition mediated by promoter activation of the pepper Bs3 resistance gene. Science. 2007;3188(1):645–648. doi: 10.1126/science.1144958. [DOI] [PubMed] [Google Scholar]
- Rusterucci C., Aviv D.H., Holt B.F., Dangl J.L., Parker J.E. The disease resistance signaling components EDS1 and PAD4 are essential regulators of the cell death pathway controlled by LSD1 in Arabidopsis. Plant Cell. 2001;138(1):2211–2224. doi: 10.1105/tpc.010085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryals J.A., Neuenschwander U.H., Willits M.G., Molina A., Steiner H., Hunt M.D. Systemic acquired resistance. Plant Cell. 1996;88(1):1809–1819. doi: 10.1105/tpc.8.10.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saraste M., Sibbald , Wittinghofer A. The P-loop-a common motif in ATP- and GTP-binding proteins. Trends Biochem. Sci. 1990;158(1):430–434. doi: 10.1016/0968-0004(90)90281-f. [DOI] [PubMed] [Google Scholar]
- Sato A., Yamamoto K.T. What's the physiological role of domain II-less Aux/IAA proteins? Plant Signal Behav. 2008;38(1):496–497. doi: 10.4161/psb.3.7.5994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schornack S., Huitema E., Cano L.M., Bozkurt T.O., Oliva R., Van Damme M., Schwizer S., Raffaele S., Chaparro-Garcia A., Farrer R., Segretin M.E., Bos J., Haas B.J., Zody M.C., Nusbaum C., Win J., Thines M., Kamoun S. Ten things to know about oomycete effectors. Mol. Plant Pathol. 2009;108(1):795–803. doi: 10.1111/j.1364-3703.2009.00593.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schornack S., Meyer A., Römer P., Jordan T., Lahaye T. Gene-for-gene-mediated recognition of nuclear-targeted AvrBs3-like bacterial effector proteins. J. Plant Physiol. 2006;1638(1):256–272. doi: 10.1016/j.jplph.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Sels J., Mathys K.J., De Coninck B.M.A., Cammue B.P.A., De Bolle M.F.C. Plant pathogenesis-related (PR) proteins: A focus on PR peptides. Plant Physiol. and Biochem. 2008;468(1):941–950. doi: 10.1016/j.plaphy.2008.06.011. [DOI] [PubMed] [Google Scholar]
- Shen K.A., Meyers B.C., Islam-Faridi M.N., Chin D.B., Stelly D.M., Michelmore R.W. Resistance gene candidates identified by PCR with degenerate oligonucleotide primers map to clusters of resistance genes in lettuce. Mol. Plant Microbe Interact. 1998;118(1):815–823. doi: 10.1094/MPMI.1998.11.8.815. [DOI] [PubMed] [Google Scholar]
- Shibuya N., Ebisu N., Kamada Y., Kaku H., Cohn , Ito Y. Localization and binding characteristics of a high-affinity binding site for N-Acetlychitooligosaccharide elicitor in the plasma membrane from suspension-cultured rice cells suggest a role as a receptor for the elicitor signal at the cell surface. Plant Cell Physiol. 1996;378(1):894–898. [Google Scholar]
- Shirasu K., Lahaye T., Tan M.W., Zhou F.S., Azevedo C., Schulze-Lefert P. A novel class of eukaryotic zinc-binding proteins is required for disease resistance signaling in barley and development in C. elegans. Cell. 1999;998(1):355–366. doi: 10.1016/s0092-8674(00)81522-6. [DOI] [PubMed] [Google Scholar]
- Shoa F., Golstein C., Ade J., Stoutemyer M., Dixon J.E., Innes R.W. Cleavage of Arabidopsis PBS1 by a bacterial type III effector. Science. 2003;3018(1):1230–1233. doi: 10.1126/science.1085671. [DOI] [PubMed] [Google Scholar]
- Simonich M.T., Innes R.W. A disease resistance gene in Arabidopsis with specificity for the avrPph3 gene of Pseudomonas syringae pv. phaseolicola. Mol. Plant Microbe Interact. 1995;88(1):637–640. doi: 10.1094/mpmi-8-0637. [DOI] [PubMed] [Google Scholar]
- Singh K., Foley R.C., Onate-Sanchez L. Transcription factors in plant defense and stress responses. Curr. Opin. Plant Biol. 2002;58(1):430–436. doi: 10.1016/s1369-5266(02)00289-3. [DOI] [PubMed] [Google Scholar]
- Spoel S.H., Dong X. Making sense of hormone crosstalk during plant immune responses. Cell Host Microbe. 2008;128(1):348–351. doi: 10.1016/j.chom.2008.05.009. [DOI] [PubMed] [Google Scholar]
- Staskawicz B.J., Dahlbeck D., Keen N.T. Cloned avirulence gene of Pseudomonas syringae pv. glycinea determines racespecific incompatibility on Glycine max (L.) Merr. Proc. Natl. Acad. Sci. USA. 1984;818(1):6024–6028. doi: 10.1073/pnas.81.19.6024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staskawicz B.J., Mudgett M.B., Dangl J.L., Galan J.E. Common and contrasting themes of plant and animal diseases. Science. 2001;2928(1):2285–2289. doi: 10.1126/science.1062013. [DOI] [PubMed] [Google Scholar]
- Stasckawicz B.J. First insights into the genes that control plant-bacterial interactions. Mol. Plant Pathol. 2009;108(1):719–720. doi: 10.1111/j.1364-3703.2009.00584.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanato F.L., Abou-Mansour E., Buchala A., Kretschmer M., Mosbach A., Hahn M., Bochet C.G., Metraux J., Schoonbeek H. The ABC transporter BcatrB from Botrytis cinerea exports camalexin and is a virulence factor on Arabidopsis thaliana. Plant J. 2009;588(1):499–510. doi: 10.1111/j.1365-313X.2009.03794.x. [DOI] [PubMed] [Google Scholar]
- Stein M., Dittgen J., Sanchez-Rodriquez C., Hou B-H., Molina A., Schulze-Lefert P., Lipka V., Somerville S. Arabidopsis PEN3/PDR8, an ATP binding cassette transporter, contributes to non-host resistance to inappropriate pathogens that enter by direct penetration. Plant Cell. 2006;188(1):731–746. doi: 10.1105/tpc.105.038372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swiderski M.R., Innes R.W. The Arabidopsis PBS1 resistance gene encodes a member of a novel protein kinase subfamily. Plant J. 2001;268(1):101–112. doi: 10.1046/j.1365-313x.2001.01014.x. [DOI] [PubMed] [Google Scholar]
- Takahashi A., Casais C., Ichimura K., Shirasu K. HSP90 interacts with RAR1 and SGT1 and is essential for RPS2-mediated disease resistance in Arabidopsis. Proc. Natl. Acad. Sci. USA. 2003;1008(1):11777–11782. doi: 10.1073/pnas.2033934100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao Y., Xie Z., Chen W., Glazebrook J., Chang H.S., Han B., Zhu T., Zou G., Katagiri F. Quantitative nature of Arabidopsis responses during compatible and incompatible interactions with the bacterial pathogen Pseudomonas syringae. Plant Cell. 2003;158(1):317–330. doi: 10.1105/tpc.007591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temple B.R., Jones A.M. The plant heterotrimeric G-protein complex. Annu. Rev. Plant Biol. 2007;588(1):249–266. doi: 10.1146/annurev.arplant.58.032806.103827. [DOI] [PubMed] [Google Scholar]
- Terras F.R., Torrekens S., Van Leuven F., Osborn R.W., Vanderleyden J., Cammue B.P.A., Broekaert W.F. A new family of basic Cysteine-rich plant antifungal proteins from Brassicaceae species. FEBS Lett. 1995;3168(1):233–240. doi: 10.1016/0014-5793(93)81299-f. [DOI] [PubMed] [Google Scholar]
- Thomma B.P., Eggermont K., Tierens K.F., Broekaert W.F. Requirement of functional ethylene-insensitive 2 gene for efficient resistance of Arabidopsis to infection by Botrytis cinerea. Plant Physiol. 1999;1218(1):1093–1102. doi: 10.1104/pp.121.4.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomma B.P., Penninckx I.A., Broekaert W.F., Cammue B.P. The complexity of disease signaling in Arabidopsis. Curr. Opin. Immunol. 2001;138(1):63–68. doi: 10.1016/s0952-7915(00)00183-7. [DOI] [PubMed] [Google Scholar]
- Tian M., Chaudhry F., Ruzicka D.R., Meagher R.B., Staiger C.J., Day B. Arabidopsis Actin-depolymerizing factor AtADF4 mediated defense signal transduction triggered by the Pseudomonas syringae effector AvrPphB. Plant Physiol. 2009;1508(1):815–824. doi: 10.1104/pp.109.137604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ting J.P., Kastner D.L., Hoffman H.M. CATERPILLERs, pyrin and hereditary immunological disorders. Nat. Rev. Immunol. 2006;68(1):183–195. doi: 10.1038/nri1788. [DOI] [PubMed] [Google Scholar]
- Tör M., Gordon P., Cuzick A., Eulgem T., Sinapidou E., Mert-Turk F., Can C., Dangl J.L., Holub E.B. Arabidopsis SGT1b is required for defense signaling conferred by several downy mildew resistance genes. Plant Cell. 2002;148(1):993–1003. doi: 10.1105/tpc.001123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tornero P., Merritt P., Sadanandom A., Shirasu K., Innes R.W., Dangl J.L. RAR1 and NDR1 contribute quantitatively to disease resistance in Arabidopsis, and their relative contributions are dependent on the R gene assayed. Plant Cell. 2002;148(1):1005–1015. doi: 10.1105/tpc.001032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuda K., Glazebrook J., Katagiri F. The interplay between MAMP and SA signaling. Plant Signal. Behav. 2008;38(1):359–361. doi: 10.4161/psb.3.6.5702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuji J., Jackson E.P., Gage D.A., Hammerschmidt R., Somerville S.C. Phytoalexin accumulation in Arabidopsis thaliana during the hypersensitive reaction to Pseudomonas syringae pv syringae. Plant Physiol. 1992;988(1):1304–1309. doi: 10.1104/pp.98.4.1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Acerveken G., Marois E., Bonas U. Recognition of the bacterial avirulence protein AvrBs3 occurs inside the host plant cell. Cell. 1996;878(1):1307–1316. doi: 10.1016/s0092-8674(00)81825-5. [DOI] [PubMed] [Google Scholar]
- Van der Biezen E.A., Jones J.D. Plant disease-resistance proteins and the gene-for-gene concept. Trends Biochem Sci. 1998;238(1):454–456. doi: 10.1016/s0968-0004(98)01311-5. [DOI] [PubMed] [Google Scholar]
- Van der Hoorn R.A., De Wit P.J., Joosten M.H. Balancing selection favors guarding resistance proteins. Trends Plant Sci. 2002;78(1):67–71. doi: 10.1016/s1360-1385(01)02188-4. [DOI] [PubMed] [Google Scholar]
- Van Loon L.C., Rep M., Pieterse C.M.J. Significance of inducible defense-related proteins in infected plants. Annu. Rev. Phytopathol. 2006;448(1):135–162. doi: 10.1146/annurev.phyto.44.070505.143425. [DOI] [PubMed] [Google Scholar]
- Van Loon L.C., Van Strien E.A. The families of pathogenesis-related proteins, their activities, and comparative analysis of PR-1 type proteins. Physiol. and Mol. Plant Pathol. 1999;558(1):85–97. [Google Scholar]
- VanEtten H.D., Mansfield J.W., Bailey J.A., Farmer E.E. Two classes of plant antibiotics: Phytoalexins versus “Phytoanticipins”. Plant Cell. 1994;68(1):1191–1192. doi: 10.1105/tpc.6.9.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan J., Zhang X.C., Stacey G. Chitin signaling and plant disease resistance. Plant Signal. Behav. 2008;38(1):831–833. doi: 10.4161/psb.3.10.5916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Pajerowska-Mukhtar K., Culler A.H., Dong X. Salicylic acid inhibits pathogen growth in plants through repression of the auxin signaling pathway. Curr. Biol. 2007;178(1):1784–1790. doi: 10.1016/j.cub.2007.09.025. [DOI] [PubMed] [Google Scholar]
- Whisson S.C., Boevink P.C., Moleleki L., Avrova A.O., Morales J.G., Gilroy E.M., Armstrong M.R., Grouffaud S., van West P., Chapman S., Hein I., Toth I.K., Pritchard L., Birch P.R. A translocation signal for delivery of oomycete effector proteins into host plant cells. Nature. 2007;4508(1):115–118. doi: 10.1038/nature06203. [DOI] [PubMed] [Google Scholar]
- Wiermer M., Feys B.J., Parker J.E. Plant immunity: the EDS1 regulatory node. Curr Opin. Plant Biol. 2005;88(1):383–389. doi: 10.1016/j.pbi.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Wilton M., Subramaniam R., Elmore J., Felsensteiner C., Coaker G., Desveaux D. The type III effector HopF2Pto targets Arabidopsis RIN4 protein to promote Pseudomonas syringae virulence. Proc. Natl. Acad. Sci. USA. 2010;1078(1):2349–2354. doi: 10.1073/pnas.0904739107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang B., Sugio A., White F.F. Avoidance of host recognition by alterations in the repetitive and C-terminal regions of AvrXa7, a type III effector of Xanthomonas oryzae pv. oryzae. Mol. Plant Microbe Interact. 2005;188(1):142–149. doi: 10.1094/MPMI-18-0142. [DOI] [PubMed] [Google Scholar]
- Yang B., Sugio A., White F.F. Os8N3 is a host disease-susceptibility gene for bacterial blight of rice. Proc. Natl. Acad. Sci. USA. 2006;1038(1):10503–10508. doi: 10.1073/pnas.0604088103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu D., Chen C., Chen Z. Evidence for an important role of WRKY DNA binding proteins in the regulation of NPR1 gene expression. Plant Cell. 2001;138(1):1527–1540. doi: 10.1105/TPC.010115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou T., Wang Y., Chen J.Q., Araki H., Jing Z., Jiang K., Shen J., Tian D. Genome-wide identification of NBS genes in japonica rice reveals significant expansion of divergent non-TIR NBS-LRR genes. Mol Genet. Genomics. 2004;278(1):402–415. doi: 10.1007/s00438-004-0990-z. [DOI] [PubMed] [Google Scholar]
- Zhu W., Yang B., Chittoor J.M., Johnson L.B., White F.F. AvrXa10 contains an acidic transcriptional activation domain in the functionally conserved C terminus. Mol. Plant Microbe Interact. 1998;118(1):824–832. doi: 10.1094/MPMI.1998.11.8.824. [DOI] [PubMed] [Google Scholar]
- Zipfel C., Robatzek S., Navarro L., Oakeley E.J., Jones J.D.G., Felix G., Boller T. Bacterial disease resistance in Arabidopsis through flagellin perception. Nature. 2004;4288(1):764–767. doi: 10.1038/nature02485. [DOI] [PubMed] [Google Scholar]
- Zipfel C. Early molecular events in PAMP-triggered immunity. Curr. Opin. Plant Biol. 2009;128(1):414–420. doi: 10.1016/j.pbi.2009.06.003. [DOI] [PubMed] [Google Scholar]