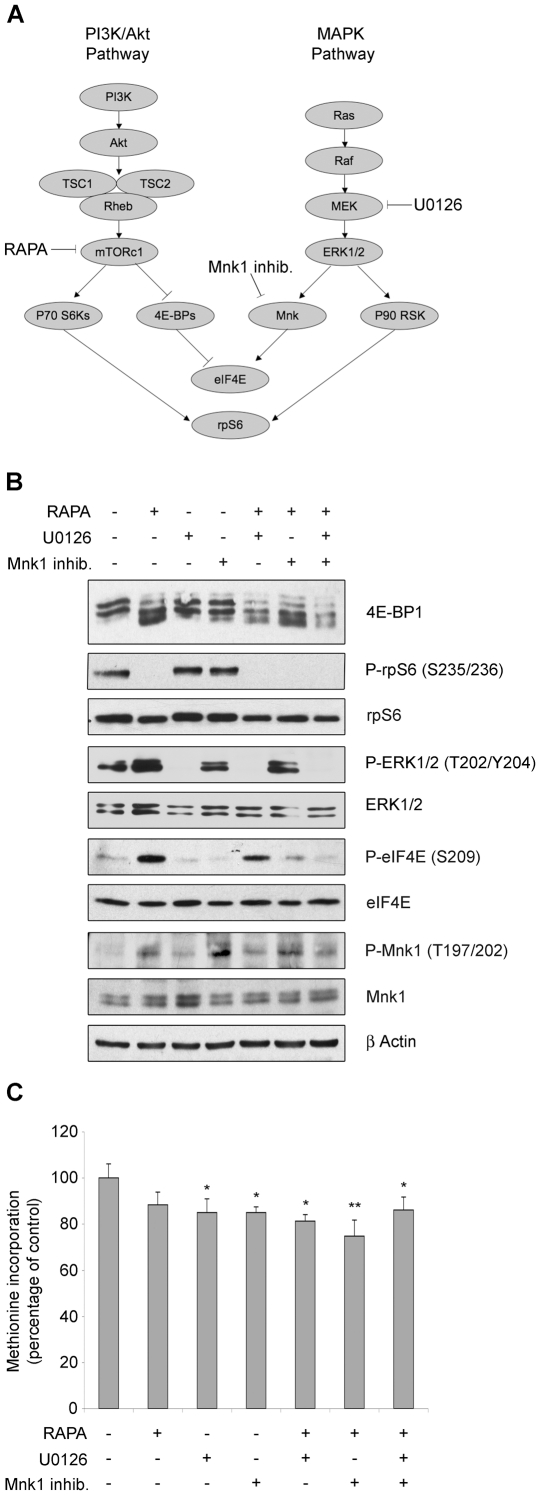
Figure 2. Translation rate is largely insensitive to the efficient inhibition of mTORc1.
(A) Scheme of major linear pathways regulating translation. (B) REN cells were treated for 45 minutes with 50 nM rapamycin (RAPA), 10 µM U0126, 5 µM 4-Amino-5-(4-fluoroanilino)-pyrazolo[3,4-d]pyrimidine (Mnk1 inhib.) as indicated and total proteins were extracted. Total extracts were analyzed by WB to test activity of mTORc1, ERK1/2 and Mnk1 signaling. Rapamycin blocks mTORc1 activity, preventing 4E-BP1 and rpS6 phosphorylation. U0126 inhibits MEK, preventing phosphorylation of downstream targets ERK1/2, Mnk1 and eIF4E. Mnk1 inhibitor 4-Amino-5-(4-fluoroanilino)-pyrazolo[3,4-d]pyrimidine blocks eIF4E phosphorylation. (C) REN cells were treated as indicated and pulsed with 35S-methionine. Methionine incorporation in newly translated proteins was measured in triplicate. mTOR, ERK1/2, or Mnk1 inactivation minimally reduce translation. Even combination of multiple drugs, in order to switch off multiple signaling pathways, has no additive effect on reducing protein synthesis.