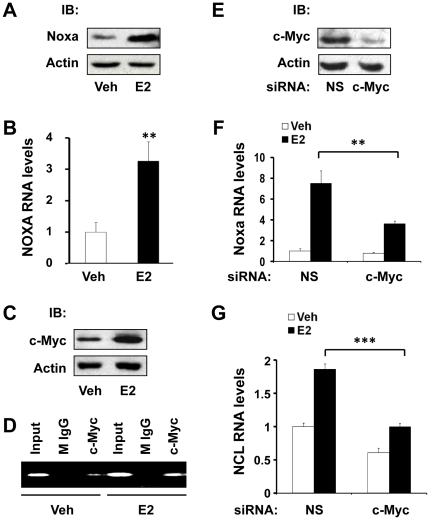
Figure 1. c-Myc mediates E2-induced Noxa transcription in human breast cancer cells.
(A–C) MCF7 cells were treated with vehicle (veh) or E2 (10 nM) for 16 hr and then harvested for analysis of Noxa protein expression by western blotting (A), Noxa mRNA expression by qPCR (B), and c-Myc protein expression by western blotting (C). (D) MCF7 cells were treated with vehicle (veh) or E2 for 4 hr, and ChIP assays using anti-c-Myc antibody or normal mouse IgG (M IgG, antibody control) were performed to analyze the effect of E2 on the recruitment of c-Myc to the NOXA promoter. Immunoprecipitated ChIP DNA was analyzed by PCR using site-specific primers that amplify a region of the NOXA promoter that contains a c-Myc binding site at +85 bp. (E) MCF7 cells were transfected with non-silencing control (NS) or c-Myc siRNA for 48 hr and then harvested for analysis of c-Myc protein levels by western blotting. (F, G) MCF7 cells were transfected with non-silencing control (NS) or c-Myc siRNA for 24 hr, followed by treatment with vehicle (veh) or E2 (10 nM) for 8 hr, and the relative mRNA expression levels of Noxa (F) and Ncl (G) were analyzed by qPCR. Graphical data points in B, F, and G are means ± S.D. of three independent experiments (** P<0.01, *** P<0.001).