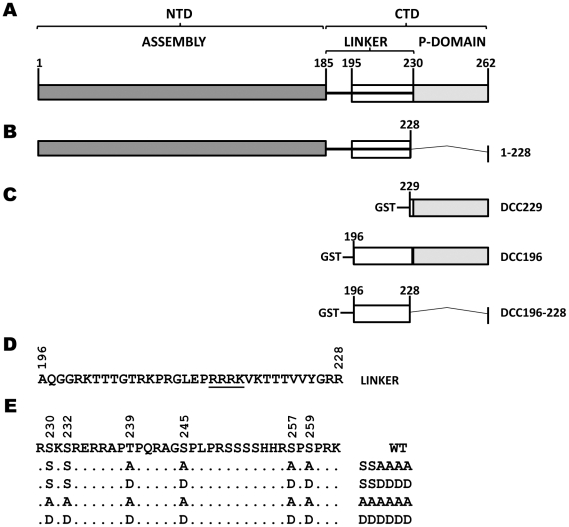
Figure 1. Schematic diagram of DHBc domains and GST-DHBc CTD (DCC) fusion constructs.
A. DHBc contains an N-terminal assembly domain (NTD) from amino acids 1–185, a flexible linker region from amino acid 186 that extends into the CTD, which includes the phosphodomain from 230–262. B. 1–228, a CTD-deletion mutant. C. Each WT fusion protein contains a differing length of the CTD and was named for the amino acid position where the tail fragment begins (229 or 196). DCC229 contains the phosphodomain from residue 229 with all six known WT phosphorylation sites (S230, S232, T239, S245, S257, and S259, panel E). DCC196 contains the phosphodomain plus the additional upstream 33 amino acids of the linker region. DCC196-228 contains amino acid residues 196–228 (the linker region), deleting the entire phosphodomain with all six phosphorylation sites. D. The linker sequence with the basic cluster underlined. E. Alanine (A) or aspartic acid (D) substitutions were made in the full-length DHBc, as well as in the context of each WT fusion protein, with the resultant mutants the same length as the WT version. The full-length A and D mutants were named by the positions of the mutated phosphosites they contain: SSAAAA, SSDDDD, AAAAAA, and DDDDDD. The GST-DCC fusion proteins were named by their amino acid starting position and their phosphosite substitutions. The final four phosphorylation sites were substituted by A in 229-SSAAAA or D in 229-SSDDDD. 196-SSAAAA contained amino acids 196–262 with the last four phosphorylation sites mutated to A and maintained S230 and S232. Similarly, 196-SSDDDD maintained S230 and S232, while the last four phosphosites contained D substitutions. Finally, all six phosphorylation sites were substituted by A in 196-AAAAAA or D in 196-DDDDDD.