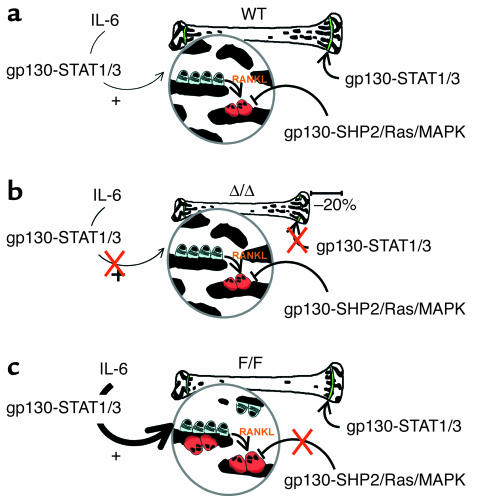
Figure 6.
Summary of the effects of gp130 signaling pathway mutations on bone structure and bone cell function. (a) In WT mice, IL-6 stimulates osteoblast proliferation via the gp130-STAT1/3 pathway, and the osteoblasts in turn stimulate osteoclastogenesis by the RANKL pathway. Osteoclastogenesis is also inhibited by gp130 family cytokines, acting through the gp130-SHP2/Ras/MAPK signaling pathway within the hematopoietic lineage. Chondrocyte proliferation is stimulated by gp130 family cytokines through the gp130-STAT1/3 pathway. (b) In the absence of the gp130-induced STAT1/3 signaling pathway, indicated by the red X (in Δ/Δ mice), chondrocyte proliferation is no longer stimulated through the gp130-STAT1/3 pathway, leading to reduced bone length. Inhibition of osteoclastogenesis through the gp130-SHP2/Ras/MAPK pathway remains intact, and while IL-6 stimulation of osteoblast differentiation can no longer occur through the gp130-STAT1/3 pathway, this is clearly a minor pathway not required for the normal level of bone formation, as bone turnover and bone structure remain at the same level as in WT mice. (c) In F/F mice, IL-6-stimulated osteoblast proliferation via gp130-STAT1/3 is enhanced, leading to a greater number of osteoblasts. Deletion of the gp130-SHP2/Ras/MAPK pathway, indicated by the red X, releases inhibition, resulting in increased osteoclastogenesis. The increase in resorption appears to be greater than the increase in bone formation, leading overall to a reduction in trabecular bone density.