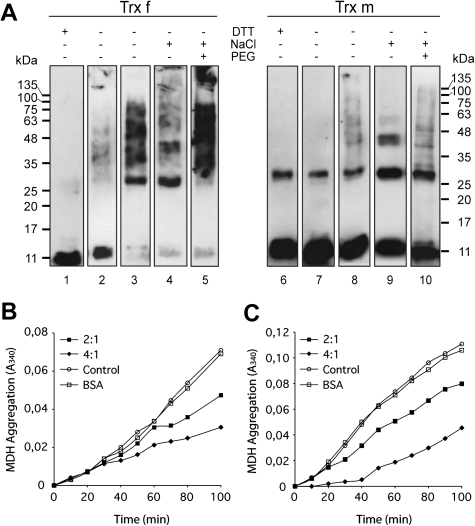
Fig. 5.
Structural changes of chloroplastic tobacco Trx f and Trx m in vitro lead chaperone holdase activity. (A) Structural changes of Trx f and Trx m, incubated in the presence or absence of DTT, NaCl or PEG 20 000, were analysed by Western blot on 13% non-reducing SDS-PAGE gels. Reduced Trxs (lanes 1 and 6), oxidized Trxs (lanes 2 and 7), oxidized Trxs incubated for 3 h at 50 °C (lanes 3 and 8), oxidized Trxs in the presence of 1 M NaCl (lanes 4 and 9), and oxidized Trxs in the presence of 150 mM NaCl and 100 mg ml−1 PEG 20 000 (lanes 5 and 10). (B, C) Holdase chaperone activity of Trx f and Trx m under different oligomeric conditions. Thermal aggregation of 2 μM MDH was examined at 43 °C in the absence (open circles) or presence of Trxs. Trx to MDH molar ratio of 2:1 (closed squares) or 4:1 (closed diamonds) were used. Bovine serum albumin (BSA, 8 μM) was used as the protein control (open squares). (B) 2 μM MDH in HEPES-KOH pH 7.5 was incubated at 43 °C in the presence of 150 mM NaCl. (C) 2 μM MDH in HEPES-KOH pH 7.5 was incubated at 43 °C in the presence of 150 mM NaCl and 100 mg ml−1 PEG 20 000. A representative plot of three independent experiments is shown.