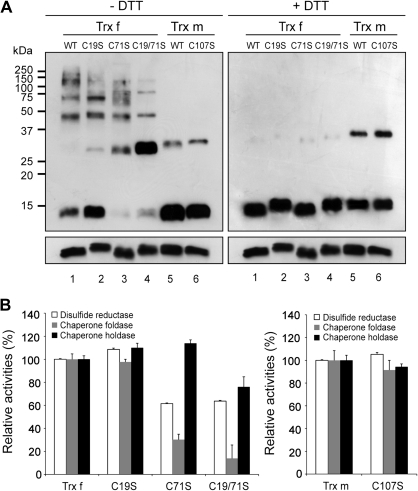
Fig. 7.
Effect of additional Cys residues on function and protein structure of chloroplastic tobacco Trx f and Trx m. (A) Redox-dependent structural changes of wild-type (WT) and mutant Trxs containing the site-specific replacement of additional Cys residues by Ser (Trx f mutants: C19S, C71S, and C19/71S; Trx m mutant: C107S). Proteins were analysed by Western blot on 13% non-reducing SDS-PAGE in the absence (left panel) or presence (right panel) of DTT. (B) Relative disulphide reductase (white bar), chaperone foldase (grey bar) and chaperone holdase (black bar) activities of the wild-type and Cys-mutant proteins. Activities of the mutants were compared with those of wild-type Trxs. The wild-type Trx f (left panel) or Trx m (right panel) activities were measured at A650 (DTT-dependent insulin reduction activity) and A340 (chaperone foldase and holdase activities) under the assay conditions described above and set to 100%, respectively. Each value is the mean ±SE (bars) of three independent determinations.