Figure 3.
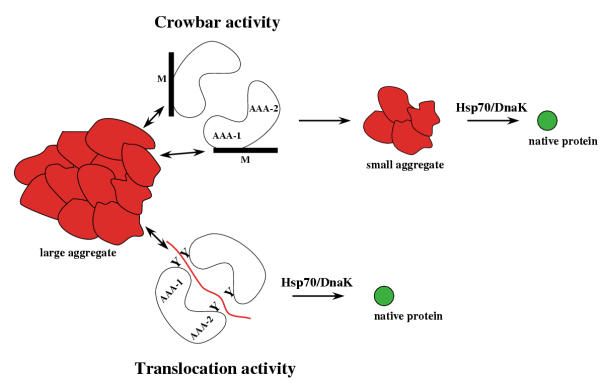
Potential mechanisms of protein disaggregation by Hsp104/ClpB. Hsp104/ClpB may break large aggregates into smaller species via a crowbar activity, mediated by movements of M-domains. Small-sized aggregates serve as substrates for Hsp70/DnaK in a sequential reaction. Hsp104/ClpB may also extract unfolded proteins from an aggregate via a translocation activity with major contributions of pore-located aromatic residues (Y). Hsp70/DnaK may directly take over the translocated polypeptide, thereby preventing the re-aggregation of extracted proteins and ensuring substrate refolding. Both suggested Hsp104/ClpB activities are not mutually exclusive, but rather may act sequentially or in concert.
