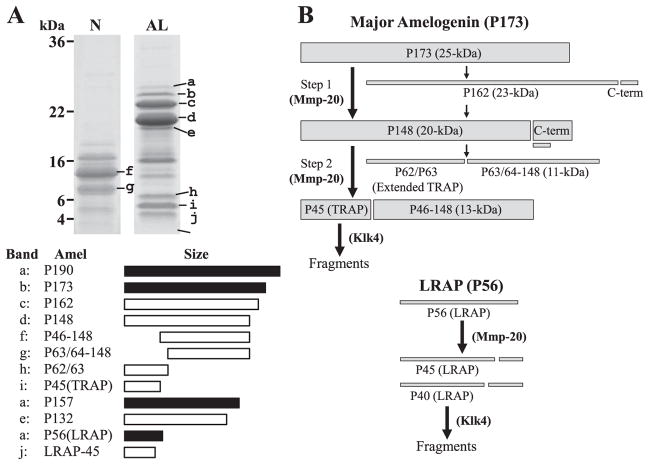
Fig. 2. Porcine amelogenin isoforms and cleavage products, and proteolytic processing pathway.
Based on the dissociative extraction method, ten amelogenin isoforms and their proteolytic cleavage products are observed on SDS-PAGE gel (N : extracts by Sorensen buffer (pH 7.4), AL : extracts by carbonate buffer (pH 10.8)). Of these amelogenins, P173 amelogenin isoform (b : P173) is a major amelogenin and its cleavage products (c : P162, d : P148, f : P46–148, g : P63/64–148, h : P62/63, i : P45 (TRAP)) are abundant during the secretory stage of enamel. The intact LRAP is found in the most outer layer enamel, but its cleavage product (j : LRAP-45) is usually detected in the mixture of outer and inner layer of enamel sample. P157 amelogenin isoform lacking exon 3 has not been identified, but its cleavage product (e : P132) has been characterized. P190 amelogenin isoform containing exon 4 encoded segment (a : P190) is the least abundant amelogenin. The major secreted amelogenin (P173) is usually processed in two steps (Fig. 2B). The initial cleavage is after Ser148 and generates P148, the most abundant amelogenin cleavage product in the matrix. The second cleavage is after Trp45 and generates tyrosine-rich amelogenin polypeptide (TRAP or P45) having an apparent molecular weight of 6-kDa and the 13-kDa amelogenins (P46–148). A minor processing pathway involves an initial cleavage after Pro162, generating the 23-kDa amelogenin (P162), which is cleaved again to generate P148. P148 is also cleaved in a less-often-used alternative pathway that generates extended TRAP having an apparent molecular weight of 7-kDa (P62/P63) and the 11-kDa amelogenin (P63/64–148). All these processing are caused by Mmp20 and Mmp20 showed little to no activity against TRAP LRAP (P56) is cleaved after Pro45 or Pro40 by Mmp20 to generate P45 (LRAP-45) and P40 (LRAP-40), and Mmp20 can not degrade any sites of LRAP other than them. The generated TRAP LRAP45 and LRAP-40 are fragmentated by Klk4 during the transition and maturation stage.