Figure 1.
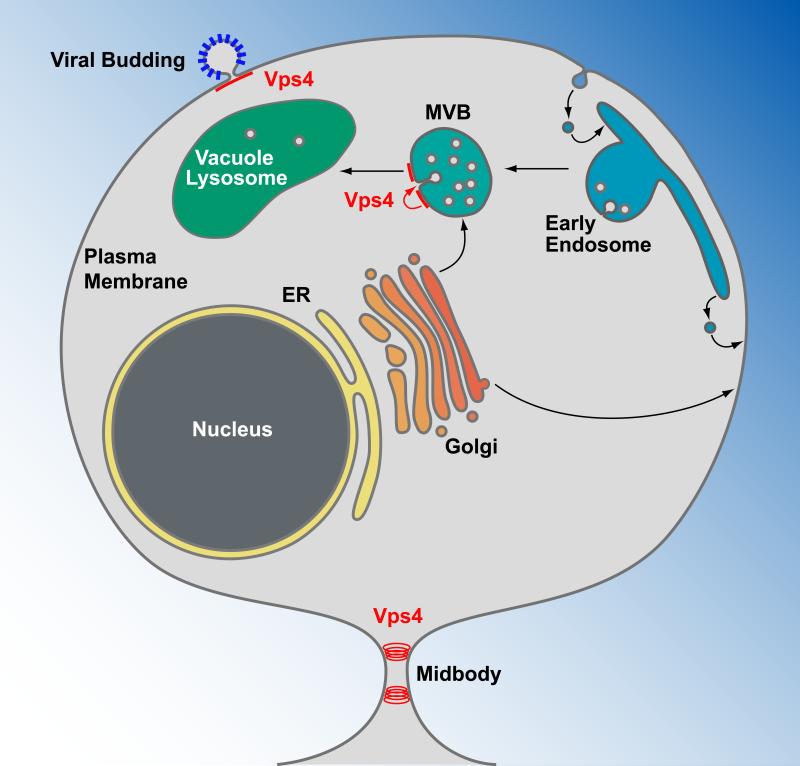
Vps4 functions in the formation of MVB vesicles, viral budding and cytokinesis. Transmembrane proteins are synthesized at the endoplasmic reticulum (ER), transported through the Golgi network and delivered either to the plasma membrane or an endosomal compartment. Plasma membrane proteins destined for degradation are endocytosed and delivered to endosomes. At the multivesicular body (MVB), these proteins are sorted by Vps4 and the ESCRTs into MVB vesicles (red arrow). Upon fusion of the MVB with the vacuole/lysosome, the lumenal vesicles are exposed to a hydrolytic environment, causing the degradation of proteins and lipids of the vesicles. Vps4 together with a subset of the ESCRTs are recruited to the plasma membrane by newly forming enveloped viruses, such as HIV-1, where they function in the release of the virus particles. At the final stage of cytokinesis, Vps4 is recruited to the midbody where it functions together with ESCRT-III in the abscission of the membrane and the formation of two separate cells.