Abstract
Commercial low molecular weight heparins (LMWHs) are prepared by several methods including peroxidative cleavage, nitrous acid cleavage, chemical ß-elimination, and enzymatic β-elimination. The disadvantages of these methods are that strong reaction conditions or harsh chemicals are used and these can result in decomposition or modification of saccharide units within the polysaccharide backbone. These side-reactions reduce product quality and yield. Here we show the partial photolysis of unfractionated heparin can be performed in distillated water using titanium dioxide (TiO2). TiO2 is a catalyst that can be easily removed by centrifugation or filtration after the photochemical reaction takes place, resulting in highly pure products. The anticoagulant activity of photodegraded LMWH (pLMWH) is comparable to the most common commercially available LMWHs (i.e., Enoxaparin and Dalteparin). 1H NMR spectra obtained show that pLMWH maintains the same core structure as unfractionated heparin. This photochemical reaction was investigated using liquid chromatography/mass spectrometry (LC/MS) and unlike other processes commonly used to prepare LMWHs, photochemically preparation affords polysaccharide chains of reduced length having both odd and even of saccharide residues.
Keywords: Low molecular weight heparin, Photochemical depolymerization, Titanium dioxide, NMR, LC-MS
1. Introduction
Heparin is a glycosaminoglycan composed of (1→4)-linked alternating glucosamine and uronic acid residues containing O-sulfo, N-acetyl and N-sulfo groups. Unfractionated heparin consists of heterogeneous polydispersed mixture of sulfated polysaccharides ranging in molecular weight from 3,000 to 30,000, with an average molecular weight of 15,000 (1-3). Heparin, a clinical anticoagulant, binds to the serine protease inhibitor antithrombin (AT), causing it to undergo a conformational change resulting in AT inhibition of thrombin (factor IIa) and factor Xa. AT binds to the specific pentasaccharide sequence (GlcNAc/NS6S (1→4) GlcA (1→4) GlcNS3S6S (1→4) IdoA2S (1→4) GlcNS6S) in heparin (4). Inactivation of thrombin by AT requires more than 18 saccharides of heparin (molecular weight<5400), and the smaller heparin oligosaccharides are unable to accelerate the inactivation of thrombin by AT but retain their ability to catalyse the inhibition of factor Xa by AT. While being widely used as a clinical anticoagulant, heparin exhibits some undesirable side effects, such as hemorrhagic complications and heparin induced thrombocytopenia and it also has low bioavailability when administered by non-intravenous routes (5-7). Low molecular weight heparins (LMWHs) have been introduced as heparin substitutes with reduced side effects, more predictable pharmacological action, sustained antithrombotic activity, and better bioavailability (7-10). The preparation of LMWH can be achieved by the treatment of heparin with: (1) nitrous acid; (2) heparin lyase; (3) hydrogen peroxide; or (4) benzyl halide and base (11-19). These methods depend on strong conditions or harsh chemicals and decomposition or modification of individual saccharide residues can be taken place, resulting in the formation of undesired side products (11-22). Furthermore, alcohol precipitation or gel filtration process is required to remove toxic chemicals such as acids or heavy metals from reaction mixture, reducing the yield of LMWH. Therefore, development of a new method is required for safe, costless and easy preparation of LMWH.
Photochemical reactions, generally involving ultraviolet (UV) light-initiated oxidation reactions, are often applied for environmentally friendly treatments, including water purification, air cleaning, clean organic syntheses and disinfection. The most widely used photocatalyst is titanium dioxide (TiO2) because of its desirable properties, such as chemical stability, high catalytic activity under UV-light, low cost and most importantly its low biological toxicity. Photocatalysis using TiO2 can be performed without special equipment or the need to control temperature. Moreover, the TiO2 used as catalyst can be easily removed by filtration after the photoreaction takes place, resulting in highly pure products.
In previous studies, we have reported the depolymerization of simple, nonsulfated polysaccharides, sodium alginate, pectin and heparosan using a photochemical reaction relying on TiO2 catalyst (23-25). The controlled depolymerization of sodium alginate, pectin and heparosan were successfully accomplished and the resulting products maintained the intact core structure of alginate, pectin and heparosan. Based on these prior studies we undertook to examine the TiO2-catalysed photochemical depolymerization of the more complex sulfated polysaccharide, heparin.
2. Materials and Methods
2.1 Materials
Heparin sodium salt, Grade I-A from porcine intestinal mucosa (212 USP units/mg), Enoxaparin (Clexane), Dalteparin, (Fragmin), RD heparin (Ardeparin), OP 2123 (Fluxum) and Logiparin (LHN-1) were obtained from Sigma-Aldrich, Co. USA, Sanofi-Aventis, France, Pfizer, USA, Opocrin, Italy, Hepar Industries, USA, and Novo Industries, Denmark, respectively. A heparin oligosaccharide standard mixture was prepared by partial (30% compleat) heparin lyase I digestion of bovine lung heparin (26). TiO2 (anatase type, particle size average, 50 μm) was purchased from Wako Pure Chemical Co. (Osaka, Japan). Electrophoresis-grade acrylamide, N,N’-methylene-bis-acrylamide, sucrose, glycine, ammonium persulfate (APS), N,N,N’,N’-tetramethylenediamine (TEMED), and pre-cast 4-15% gradient mini-slab gel were from Bio-Rad, (Hercules, CA, USA). Boric acid, disodium salt of ethylenediamine tetraacetic acid (EDTA), phenol red, and Alcian blue were from Fisher (Pittsburgh, PA, USA).
2.2 Photochemical reaction
The photochemical reaction device (Sen Lights Corporation, Osaka, Japan) consisted of a VG1500 reaction tank with 5 inlets, a light source (high pressure mercury lamp HL100 CH-4, 100W), a power source (HB100P-1) and lamp jacket-quartz glass JW-2Q and Pyrex glass JW-1G. The apparatus was connected with a water circulating system to cool the lamp.
2.3 Degradation of unfractionated heparin by photochemical reaction
Unfractionated heparin (10 mg) was dissolved in 1 mL water with 1 mg of titanium dioxide (TiO2) particles in a tube (borosilicate Pyrex glass, 13 mm i.d. × 120 mm, Iwaki glass Co. Ltd., Tokyo) and loosely closed by a screw cap. The sample tube was the placed in the photochemical reaction tank and was exposed to light within 2 cm distance from the lamp unit at room temperature. A mechanical stirrer was used in addition to a magnetic stirrer to insure the dissolution of oxygen into the solution. After the reaction, the sample was centrifuged at 1500 × g for 5 min at 20°C and the supernatant was filtered through 0.45 μm disposable syringe filter unit (Dismic-13HP; Advantec, Tokyo, Japan) to eliminate all of the TiO2 particles, and the product solution was neutralized with NaOH, dialyzed and lyophilized. The change in molecular weights of degraded heparin samples were monitored by GPC-HPLC.
2.4 Estimation of the average molecular weight
GPC-HPLC was performed using a TSK-GEL G3000PWxl size exclusion column with a sample injection volume of 20 μL (20 μg) at a flow rate of 0.6 mL/min on an apparatus composed of a Shimadzu LC-10Ai pump, a Shimadzu CBM-20A controller and a Shimadzu RID-10A refractive index detector. The mobile phase consisted of 0.1 M NaNO3. The column was maintained at 40°C with an Eppendorf column heater during the chromatography. The GPC chromatograms were recorded on a computer with the LCsolution Version 1.25 software and analyzed with its “GPC Postrun” function. Heparin oligosaccharides of different molecular weights purchased from Iduron (Manchester, UK) were used as calibrants for the standard curve.
2.5 Measurement of level of sulfo group loss and glucosamine content
The glucosamine for heparin and inorganic sulfate were determined separately by high performance liquid chromatography (HPLC) after acidic hydrolysis of heparin (27). Determination of sulfate groups was performed by anion-exchange HPLC after acid hydrolysis of the sample in 2.5 M trifluoroaceteic acid (TFA) at 100°C for 2.5 h using conductively detection (Tosoh model CM-8020). The column used was TSK gel IC-Anion-PWXL (4.6 mm, i.d. × 350 mm) (Tosoh, Japan) and eluted with 1.42 mM NaHCO3 and 1.5 mM Na2CO3 at a flow rate of 0.8 mL/min. A standard calibration curve was constructed using Na2SO4. Glucosamine was determined by the post column HPLC derivatization after acid hydrolysis under identical conditions as described for sulfate analysis (28).
2.6 Structural analysis by 1H NMR
All 1D 1H-NMR experiments were performed at 298 K on Bruker Avance II 600 MHz spectrometer with Topspin 2.0 software. Heparin and LMWHs (2 mg) were each dissolved in 0.4 mL D2O (99.96%, Sigma-Aldrich, Co.) and lyophilized three-times to remove the exchangeable protons. The samples were re-dissolved in 0.4 mL D2O and transferred to NMR microtubes (OD 5 mm, Norrell). Experiments were performed with 32 scans and an acquisition time of 2.6 sec. The conditions for two-dimensional HMQC spectra were as follows: 32 scans, sweep width of 6.15 kHz, acquisition time of 0.33 sec, and relaxation delay of 0.90 sec. The conditions for two-dimensional COSY spectra were as follows: 8 scans, sweep width of 7.40 kHz, acquisition time of 0.28 sec, and relaxation delay of 1.50 sec.
2.7 Polyacrylamide electrophoresis
Polyacrylamide gel was prepared in 22% of total acrylamide for resolving gel (0.75 mm thick × 6.8 cm × 8.6 cm) and 5% of total acrylamide for stacking gel. Upper chamber and lower chamber were filled with buffer A (0.2 M Tris (pH 9 without adjustment) and 1 M glycine) and buffer B (0.1 M Tris-HCl (pH 8.3), 0.1 M boric acid 10 mM EDTA), respectively. LMWH sample was diluted with equivalent volume of 50% sucrose and phenol red was added to the sample for the visualization. The gel was loaded with 7.5 μg of each LMWH sample and subjected to electrophoresis for 80 min at 200 V. The gel was visualised first with 0.5% (w/v) Alcian blue in 2% (v/v) acetic acid aqueous solution.
2.8 Preparation of LMWH by heparin lyase and ozonolysis
Flavobacterium heparin lyases I, II and III were prepared as described previously (29, 30). A reaction mixture (3 mL), contained 25 mM Tris HCl (pH 7.4), 500 mM NaCl, 10 mg of Heparin from porcine intestinal mucosa, 3 U of heparin lyases I, II, and III were incubated 3.5 h at room temperature. After incubation, the reactions was stopped by boiling for 5 min and centrifuged for 5 min at 3,000 × g. The extent of depolymerization was determined by measuring the A232 using a Nanodrop ND-1000 spectrophotometer (Thermo Fisher Scientific Inc., USA). The extent of depolymerization for heparin was approximately 70% respectively. For ozonolysis, a mixture of heparin oligosaccharide standards was diluted in water at 0.2 mg/mL. Ozone, generated from a Welsbach model T-816 (El Sobrante, CA) apparatus set at 0.4 L/min (O2 at 21°C) air-flow and 70 V, was bubbled through the solution for 5 min. The reaction was worked up by adjusting the sample to pH 3 with 1 M hydrochloric acid and incubating for 30 min at 37° C. The sample was adjusted to pH 7 with 10% aqueous sodium hydroxide. The sample was desalted by 3000 molecular weight cut-off (MWCO) spin column (Millipore, USA) and lyophilized.
2.9 Liquid chromatography-mass spectrometric analysis of photodegraded low molecular weight heparin
LC-MS analyses were performed on an Agilent 1100 LC/MSD instrument (Agilent Technologies, Inc. Wilmington, DE) equipped with an ion trap, binary pump followed by microflow, and a UV detector. The sample (8 μL, 50 mg/mL) was injected. The column used was a 1.7 μm Acquity UPLC BEH C18 column (2.1 × 150 mm, Waters, Milford, MA, USA) (31). For HP oilgosaccharides analysis, solutions A and B for UPLC were 0 and 75% acetonitrile, respectively, containing the same concentration of 15 mM n-hexylamine (HXA) as an ion-pairing reagent and 100 mM 1,1,1,3,3,3-hexafluoro-2-propanol (HFIP) as an organic modifier. The column temperature was maintained at 45 °C. Solution A for 10 min, followed by a linear gradient from 10 to 120 min of 0 to 80% solution B at the flow rate of 100 μL/min was used for oilgosaccharides analysis. The electrospray interface was set in positive ionization mode with the skimmer potential 40.0 V, capillary exit 40.0 V, and a source of temperature of 350 °C to obtain maximum abundance of the ions in a full-scan spectra (350–2000 Da, 10 full scans/s). Nitrogen was used as a drying gas (8 L/min) and a nebulizing gas (40 psi).
2.10 Anticoagulation activity of heparin, pLMWH, Dalteparin and enoxaparin
Anti-factor Xa activity was measured using Test Team® heparin S kit (Sekisui Medical, Tokyo, Japan). A reaction mixture 1 (0.2 mL), contained 50 mM Tris HCl (pH 8.4), 20 mL of human plasma, 20 mU of AT and 10 ng of unfractionated heparin and LMWH were incubated 37°C at 2 min. After incubation, reaction mixture 2 (0.3 mL), contained 42.6 mU of factor Xa and 150 μg of S-2222, was added and incubated at 3 min. Reaction was stopped with 300 μL of 8.33 M acetic acid and absorbance at 405 nm was measured. Anti-thrombin activity was measured using Test Team® ATIII 2 kit (Sekisui Medical, Tokyo, Japan) following the supplier's manual with minor modifications. A reaction mixture (75 μL), contained 100 mM Tris HCl (pH 7.4), 12 mU of thrombin and 10 ng of unfractionated heparin or LMWH were incubated 37°C at 5 min. After incubation, 50 μL of 1.43 mg/mL of S-2238 were added and incubated at 5 min. Reaction was stopped using citric acid and absorbance at 405 nm was measured.
3. Results and Discussion
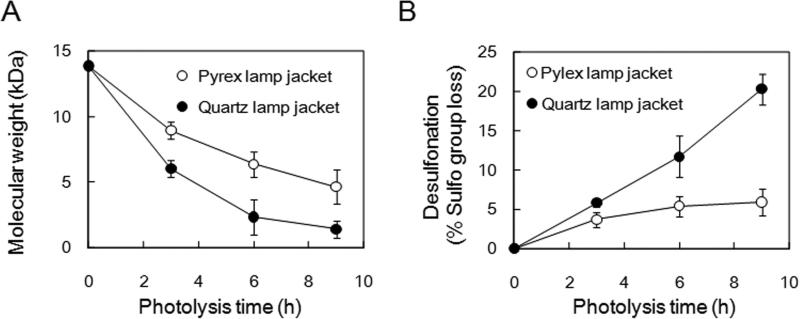
Photochemical activation of aqueous titanium dioxide (TiO2) affords to produce reactive oxygen species that are capable of randomly oxidizing the sugar residues of polysaccharides, resulting in a decrease in polysaccharide molecular weight. In previous studies, sodium alginate and pectin were depolymerized by photochemical reaction under the various pH conditions. First of all, effects of pH of reaction mixture was investigated at pH 4 (water adjusted with 10 mM hydrochloric acid), pH 7 (water adjusted with 10 mM ammonium hydroxide) and pH 10 (water adjusted with 100 mM ammonium hydroxide) and water (20, 21). The dissolved heparin (10 mg/mL), suspended with 1 mg TiO2 particles in 6 mL-screw-capped test tube (borosilicate glass), were exposed to a high-pressure mercury UV light for 0, 1, 3 and 6 h. The molecular weight of samples at each pH condition was determined by GPC-HPLC. The degrees of depolymerization of heparin at pH 4 and 7, and in water were not different each other, while heparin was easily depolymerized at pH 10 (data not shown). On the other hand, TiO2 (80% anatase, 20% rutile)-catalysed photocatalytic degradation of unfractionated heparin had previously been described using a quartz lamp jacket (125 W medium pressure mercury lamp) in the oxygen-saturated water (32). Under the condition, however, significant desulfonation and decarboxylation of the resulting LMWH products were observed. It has been reported that a mixture of anatase and rutile TiO2 nanoparticles has a much higher photocatalytic activity than pure the anatase or rutile TiO2 nanoparticles (33). We had previously demonstrated the pure anatase TiO2-catalysed photo-depolymerization of alginate and pectin, unsulfated polysaccharides using quartz lamp jacket (100 W high pressure mercury lamp, wavelength range: 254 – 436 nm) without concomitant structural damage to these molecules (23, 24). Depolymerization of heparin was examined using quart lamp jacket (100 W high pressure mercury lamp, wavelength range: 254 – 436 nm), however, significant desulfonation of heparin was observed (Fig. 1B). To depolymerize heparin without structural damages, Pyrex borosilicate glass lamp jacket was used instead of a quartz lamp jacket, because a Pyrex borosilicate glass lamp jacket is known to decrease the transmission of UV light. Thus, TiO2-catalysed photochemical reaction was performed using Pyrex glass lamp jacket (100W high pressure mercury lamp, wavelength range: >370 nm) (Fig. 1). Molecular weight of the starting heparin and the photodegraded low molecular weight heparin (pLMWH) was determined by GPC-HPLC and the desulfonation of the pLMWH was determined by anion-exchange HPLC. While the rate of depolymerization by light filtered through a Pyrex lamp jacket was slightly reduced, there was a remarkable decrease in desulfonation observed when using a Pyrex lamp jacket (Fig. 1A and 1B). Thus, the optimal conditions for a photochemical reaction with >370 nm light and for purification to obtain LMWH were established as exposure to the visible light in the presence of TiO2 in water. Heparin depolymerization by photolysis is thought to be occured via mechanisms of radical-induced scission of glycosidic linkage because photo-irradiation of TiO2 leads to the formation of active oxygen radical (H2O2, O2- and ·OH) (34-36). In the case of preparation of LMWHs from Fenton's type depolymerization process or γ-ray irradiation, not only resulting LMWH but also several side products were generated (17, 37, 38). Although depolymerization of heparin by photolysis using Pyrex glass lamp jackets was milder than Fenton reaction or γ-ray irradiation preparations (17, 37, 38), small compounds produced by photolysis were removed by dialysis against water to avoid contamination in pLMWH, and the samples were neutralized and freeze dried. After the photolytic preparation procedure of pLMWH described above, 93% yield of pLMWH was obtained from the original unfractionated heparin. The number-averaged molecular weight (MN), weight-averaged molecular weight (MW), polydispersity (P) and number of sulfate groups/disaccharide of pLMWH which obtained by the photolytic preparation were shown in Table 1. By carefully controlling heparin photolysis over the course of 12 h, we were able to obtain a pLMWH having a molecular weight comparable to commercial LMWHs. Numbers of sulfo groups of each LMWH were determined and pLMWH retained 96% of sulfo groups presented in the original unfractionated heparin.
Figure 1. Photochemical depolymerization of heparin.
A. Molecular weight is measured as a function of photolysis time. B. Desulfonation (% sulfo group loss) is measured as a function of photolysis time. Pyrex glass (o) and quartz (•) lamp jackets were evaluated. HPLC condition was performed as described under “Materials and Methods”.
Table 1.
Molecular weight (MN, MW and P) of unfractioned heparin, pLMWH, enoxaparin and dalteparin.
| MN | MW | P | Number of sulfate groups/disaccharide | |
|---|---|---|---|---|
| Heparin | 13873 | 20073 | 1.45 | 2.16 |
| pLMWH | 3503 | 5074 | 1.45 | 2.08 |
| Enoxaparin | 2633 | 3873 | 1.47 | 1.94 |
| Dalteparin | 4208 | 5635 | 1.34 | 2.35 |
We next measured anti-factor Xa and thrombin activity of heparin, pLMWH to examine whether a structure of AT-binding sequence of heparin is preserved (Table 2). Anti-factor Xa activity and thrombin activity of pLMWH were approximately 81% and 36%, respectively, of unfractionated heparin and most importantly these anticoagulant activities were comparable to those of LMWHs (Enoxaparin and Dalteparin) currently clinical use. Anti-factor Xa to anti-thrombin ratios for pLMWH, Enoxaparin and Dalteparin were 2.34, 3.24 and 3.13, respectively. The value of pLMWH seems to be suitable for clinical use.
Table 2.
Anti-Xa and Anti-thrombin activity of heparin, pLMWH, enoxaparin and dalteparin.
| Anti-Xa activity (U/mg) | Anti-thrombin activity (U/mg) | Anti-Xa/Anti-thrombin (Ratio) | |
|---|---|---|---|
| Heparin | 206.2 ± 3.10 | 208.3 ± 5.42 | 0.99 |
| pLMWH | 168.3 ± 3.22 | 72.5 ± 2.61 | 2.32 |
| Enoxaparin | 171.1 ± 3.80 | 44.1 ± 19.95 | 3.88 |
| Dalteparin | 221.5 ± 2.65 | 93.0 ± 8.94 | 2.38 |
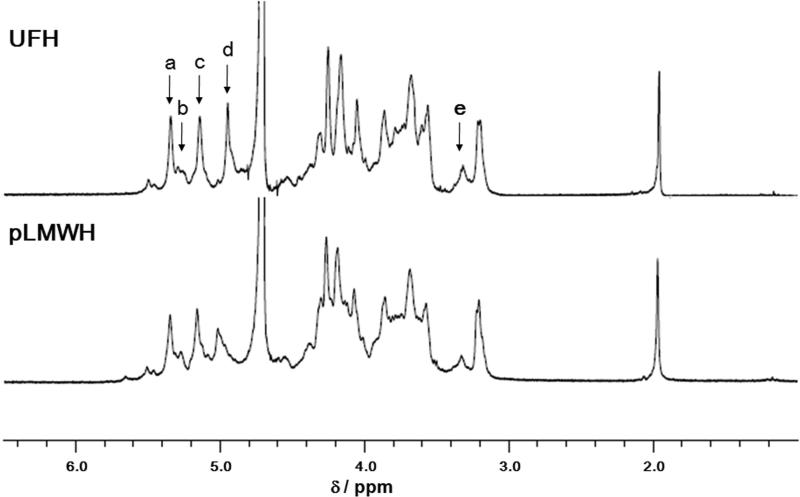
The 1H NMR spectra at 600 MHz of heparin and pLMWH are shown in Fig. 3. The peaks of the components of heparin were assigned based on the data reported previously (39) and on two-dimensional HMQC and COSY experiments (data not shown). The signals for the pLMWH sample showed the same chemical shift values and signal intensities as observed for the intact heparin sample, except for a reduced intensity IdoA H-5 peak (4.94 ppm) (Fig. 2). These results strongly suggest that depolymerization of heparin by photolysis with >370 nm light was achieved with maintaining the intact core structure of heparin.
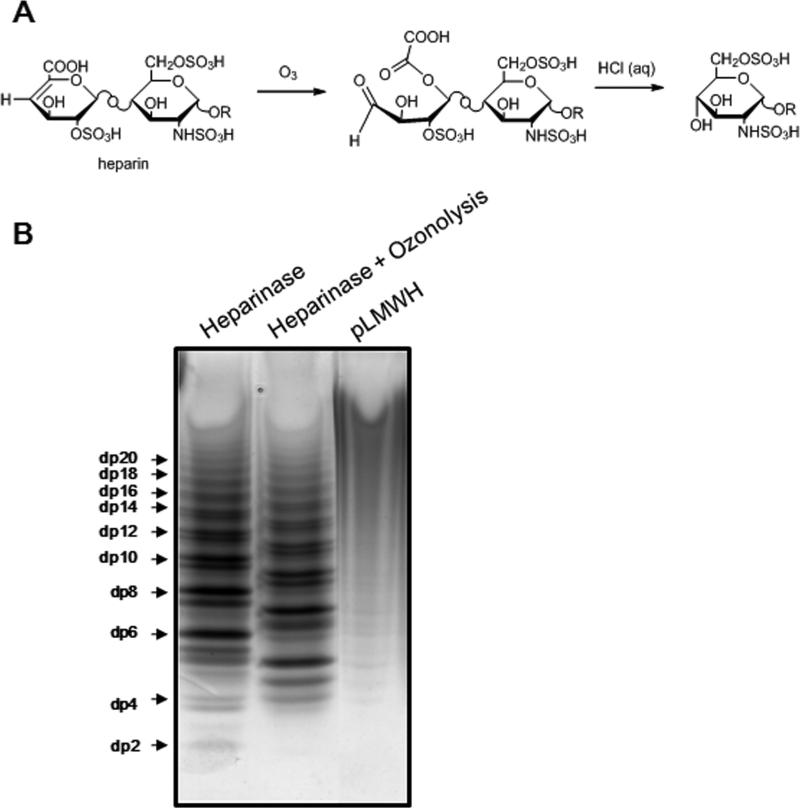
Figure 3. Removal of the unsaturated uronic acid residue of heparin by ozonolysis.
A. The proposed mechanism of ozonolysis of heparosan and heparin. In heparin R = [ → 4)-α-l-IdoA ± 2S(or β-d-GlcA)(1 → 4)-α-d-GlcNS±6Sand/or±3S(or GlcNAc±6S)(1 → ]n 4)-α-l-IdoA±2S(or β-d-GlcA)(1 → 4)-αβ-d-GlcNS±6Sand/or±3S(or GlcAc±6S). B. Preparation of LMWH by heparinase treatment followed by ozonolysis. 10 μg of LMWH prepared by heparinase, LMWH obtained by heparinase treatment followed by ozonolysis and pLMWH were separated by PAGE on a 22% acrylamide gel and stained with 0.5% (w/v) Alcian blue in 2% (v/v) acetic acid aqueous solution.
Figure 2. Proton NMR spectra of A. unfractionated heparin and B. pLMWH.
Peak assignments are: a. GlcNS6S H-1; b. GlcNS H-1 linked to IdoA; c. IdoA2S H-1; d. IdoA H-5; and e. GlcNS3S H-2 linked to GlcA.
Heparin depolymerization by active oxygen radical is occured via mechanisms of random scission of glycosidic linkage, resulting main products having both the natural reducing and non-reducing end-terminal residues (16, 37). These phenomena have been observed by the depolymerization of heparin by γ-irradiation and been investigated using MALDI mass spectrometry (17). To detect odd number oligosaccharides from pLMWH, it is important to prepare odd number heparin oligosaccharides as a standard for LC-MS analysis. It has been reported that hyaluronic oligosaccharides prepared using polysaccharide lyase was treated with ozone to confirm the presence of a double bond in the C4 and C5 position of uronic acid moiety (40). We have also previously found that an unsaturated uronate residue was successfully cleaved to form odd number of low molecular weight heparin (Fig. 3A) (41). Thus, LMWH obtained by heparin lyase treatment followed by ozonolysis were prepared and analyzed on polyacrylamide gel electrophoresis (PAGE). As shown in Fig. 3B, the resulting products by ozonolysis were polysaccharide chains having an odd number of saccharide units. As for pLMWH, distribution of oligosaccharide and polysaccharide chains afforded by photolysis showed a profile indicating that photochemical reaction and oxidative cleavage products contained oligosaccharide and polysaccharide chains having both an odd and even number of saccharide units. It has been known that OP 2123, prepared oxidative cleavage of hydroxyl radical (·OH), contains both even and odd number of saccharide units (16). To compare the molecular weight dispersity of oligosaccharide and polysaccharide chains of pLMWH with those of RD heparin and OP 2123, PAGE analysis was performed and indicating that these LMWHs showed no banding as well as pLMWH (supplementary data 1). On the other hand, Logiparin and Enoxaparin, prepared by chemical or enzymatic ß-elimination, showed profiles indicating that they consisted of oligosaccharide and polysaccharide chains having an even number of saccharide units. These data strongly support the fact that heparin depolymerization by photolysis is caused via mechanisms of random scission of glycosidic linkage, but not broken main core structure.
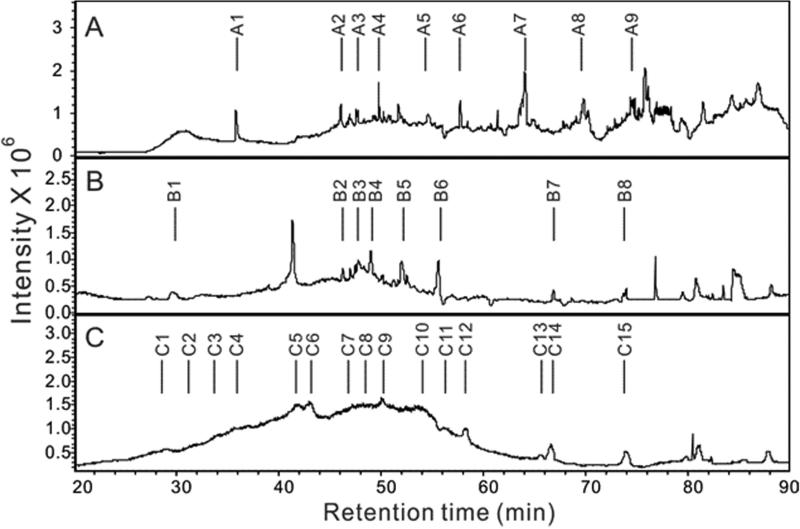
The LC-MS analysis was undertaken on pLMWH, LMWH prepared through the controlled heparin lyase catalyzed depolymerization of heparin, and LMWH prepared using heparin lyase and then subjected to ozonolysis (Fig. 4). Analysis utilized reversed phase ion pairing-ultraperformance liquid chromatography (RPIP-UPLC) for sample fractionation and ion-trap mass spectrometry for detection. The both LMWHs showed well-resolved peaks in their total ion chromatogram (TIC) (Fig. 4A and B). Analysis of the major peaks in these chromatograms show that LMWH prepared using heparin lyase is composed of chains (A1-A9) all having an even number of saccharide units and that after being subjected to ozonolysis the resulting LMWH contains only chains (B1-B8) composed of an odd number of saccharide units (Table 1). These results are expected based on the specificity of heparin lyase and the removal by ozonolysis of a single ΔUA residue at the non-reducing end of the chain. Similar analysis of pLMWH showed broad, poorly resolved peaks in the TIC consisting of chains (C1-C15) (Fig. 6C). The pLMWH contains a complex mixture of both even and odd numbered chains explaining their poor resolution on RPIP-UPLC (Table 1). Side effects of the odd number of oligosaccharide and polysaccharide chains in pLMWH, if they happen, are unlikely because it has been reported that commercial bovine lung heparin consists of both even and odd number of saccharide residues (42).
Figure 4. Total ion chromatography (TIC) of LMWH obtained by A. heparinase treatment; B. heparinase treatment followed by ozonolysis and C. photochemical depolymerization (pLMWH).
The separation was performed on an Acquity UPLC BEH C18 column (2.1×150 mm, 1.7 μm, Waters). Solutions A and B for UPLC were 0 and 75% acetonitrile, respectively, containing the same concentration of 15 mM HXA as an ion-pairing reagent and 100 mM HFIP as an organic modifier. The column temperature was maintained at 45 °C. Solution A for 10 min, followed by a linear gradient from 10 to 120 min of 0 to 80% solution B at the flow rate of 100 μL/min was used for oilgosaccharides analysis.
In summary, this study shows that the use of a Pyrex glass light filter can markedly decrease sulfo group loss in the TiO2-catalysed depolymerization of heparin. Careful control of the reaction time affords a pLMWH that has molecular weight properties and in vitro activities comparable to commercial LMWHs. Photo-depolymerization relies on mild reaction conditions, requires no harsh or toxic reagents, and affords a product free of chemical artifacts. The photolytic process might be possible for large scale production of low molecular weight heparin by the improvement of instrumentation, especially, the light sources. The reaction was to be able to scale up to 250 mL of a mixture of heparin and TiO2 by the instrument used in this study. Future studies will be required to assess the in vivo biological activities and pharmacological efficacy of pLMWH.
Supplementary Material
Supplementary Material to Higashi et al. “Photochemical preparation of a novel low molecular weight heparin”
Supplemental figure 1. Polyacrylamide gel electrophoresis of LMWHs.
Lanes contain: a. controlled heparinase depolymerization of bovine lung heparin; b. pLMWH; c, RD heparin; d, OP2123; e, Logiparin; and f. Enoxaparin. 5 μg of controlled heparinase depolymerization of bovine lung heparin (a) and 7.5 μg LMWHs (b-f) were separated by PAGE on a 22% acrylamide gel and stained with 0.5% (w/v) Alcian blue in 2% (v/v) acetic acid aqueous solution.
Highlits.
>We developed a new process for low molecular weight heparin by photolysis using titanium dioxide. >The prepared low molecular weight heparin maintains intact core structure of unfractionated heparin. >The prepared low molecular weight heparin showed similar anticoagulant behavior compared with the low molecular weight heparin drugs.
What is known on this topic
Commercial low molecular weight heparins (LMWHs) are prepared by several methods including peroxidative cleavage, nitrous acid cleavage, chemical ß-elimination, and enzymatic β-elimination.
What this paper adds
The present work describes partial photolysis of unfractionated heparin can be performed in distillated water using TiO2. TiO2 is a catalyst that can be easily removed, by centrifugation or filtration, after the photochemical reaction takes place, resulting in highly pure products.
Photogenerated low molecular weight heparin (pLMWH) retained intact core structure and anticoagulant activity was comparable to the most common commercially available LMWHs.
Table 3.
Major fractions obtained LMWH obtained by heparinase treatment (A1-10), heparinase treatment followed by ozonolysis (B1-8) and by photochemical depolymerization (C1-15)
| Fractions | Observed ionsa (charge state, z) | Composition |
Theoretical Mol Mass | |||
|---|---|---|---|---|---|---|
| Molecular ion | DPb | Monosaccharides (sulfo groups, S) | Calculated Mol Mass | |||
| A1 | 880.5 (+2) | [M+6HXA+2H]2+ | 4 | 1 ΔUA+2GlcN+1IdoA/GlcA (6S) | 1153.0 | 1153.9 |
| 980.5 (+2) | [M+8HXA]2+ | |||||
| A2 | 1030.4(+2) | [M+6HXA+2H]2+ | 6 | 1 ΔUA+2GlcN+2IdoA/GlcA+1GlcNAc (5S)c | 1452.8 | 1453.1 |
| 1080.9(+2) | [M+7HXA+2H]2+ | |||||
| A3 | 1049.2(+2) | [M+6HXA+2H]2+ | 6 | 1 ΔUA+3GlcN+2IdoA/GlcA (6S) | 1490.4 | 1491.0 |
| 1099.7(+2) | [M+7HXA+H]2+ | |||||
| A4 | 1060.5(+2) | [M+5HXA+2H]2+ | 6 | 1 ΔUA+2GlcN+2IdoA/GlcA+1GlcNAc (7S) | 1614.0 | 1613.0 |
| 1139.6(+2) | [M+7HXA+2H]2+ | 1 ΔUA+3GlcN+2IdoA/GlcA (7S) | 1570.2 | 1571.0 | ||
| A5 | 1230.4(+2) | [M+8HXA+2H]2+ | 6 | 1 ΔUA+3GlcN+2IdoA/GlcA (8S) | 1650.8 | 1651.0 |
| A6 | 790.7(+4) | [M+10HXA+4H-S]4+ | 8 | 1 ΔUA+4GlcN+3IdoA/GlcA (11S) | 2228.8 | 2227.9 |
| 835.8(+4) | [M+11HXA+4H]4+ | 2228.2 | ||||
| A7 | 764.2(+5) | [M+10HXA+5H]5+ | 10 | 1 ΔUA+5GlcN+4IdoA/GlcA (14S) | 2806.0 | 2805.0 |
| 723.8(+5) | [M+8HXA+5H]5+ | |||||
| A8 | 844.5(+6) | [M+17HXA+5H]6+ | 12 | 1 ΔUA+5GlcN+5IdoA/GlcA+1GlcNAc (16S) | 3345.0 | 3343.9 |
| 1020.5(+5) | [M+17HXA+5H]5+ | 1 ΔUA+6GlcN+5IdoA/GlcA (17S) | 3380.5 | 3381.9 | ||
| A9 | 893.8(+6) | [M+15HXA+6H]6+ | 14 | 1 ΔUA+6GlcN+6IdoA/GlcA+1GlcNAc (18S) | 3841.8 | 3840.9 |
| 933.9 (+6) | [M+17HXA+6H]6+ | 1 ΔUA+7GlcN+6IdoA/GlcA (19S) | 3880.4 | 3879.0 | ||
| 980.7 (+6) | [M+19HXA+6H]6+ | 1 ΔUA+7GlcN+6IdoA/GlcA (20S) | 3959.2 | 3958.8 | ||
| B1 | 899.9(+1) | [M+HXA+H]1+ | 3 | 1GlcN+1IdoA/GlcA+1 GlcNAc (3S) | 797.9 | 798.1 |
| B2 | 991.7(+2) | [M+6HXA+2H]2+ | 5 | 2GlcN+2IdoA/GlcA+1GlcNAc (6S) | 1375.4 | 1375.0 |
| B3 | 1082.4(+2) | [M+7HXA+2H]2+ | 5 | 2GlcN+2IdoA/GlcA+1GlcNAc (7S) | 1455.8 | 1455.0 |
| B4 | 1061.1(+2) | [M+7HXA+2H]2+ | 5 | 3GlcN+2IdoA/GlcA (7S) | 1413.2 | 1413.0 |
| B5 | 1152.0(+2) | [M+8HXA+2H]2+ | 5 | 3GlcN+2IdoA/GlcA (8S) | 1494.0 | 1493.0 |
| B6 | 1067.9 (+3) | [M+12HXA+H]3+ | 7 | 4GlcN+3IdoA/GlcA (10S) | 1990.7 | 1990.0 |
| B7 | 763.9 (+6) | [M+15HXA+6H]6+ | 11 | 6GlcN+5IdoA/GlcA (15S) | 3062.4 | 3063.9 |
| 726.7 (+6) | [M+12HXA+6H]6+ | 6GlcN+5IdoA/GlcA (16S) | 3142.2 | 3143.9 | ||
| B8 | 622.7(+8) | [M+12HXA+8H]8+ | 13 | 6GlcN+6IdoA/GlcA+1GlcNAc (19S) | 3761.6 | 3762.9 |
| 660.5(+8) | [M+17HXA+8H]8+ | 7GlcN+6IdoA/GlcA (17S) | 3559.0 | 3560.8 | ||
| 847.0(+7) | [M+21HXA+6H]7+ | 7GlcN+6IdoA/GlcA (20S) | 3802.0 | 3800.8 | ||
| C1 | 894.4(+1) | [M+2HXA+H]1+ | 3 | 1GlcN+2IdoA/GlcA(2S) | 691.4 | 691.0 |
| 814.4(+1) | [M+2HXA+H-S]1+ | |||||
| 716.5(+1) | [M+HXA+H-S]1+ | |||||
| C2 | 899.9(+1) | [M+HXA+H]1+ | 3 | 1GlcN+1IdoA/GlcA+1 GlcNAc (3S) | 797.9 | 798.1 |
| C3 | 899.7(+2) | [M+7HXA]2+ | 4 | 2GlcN+2IdoA/GlcA (5S) | 1092.4 | 1092.0 |
| 880.8(+2) | [M+7HXA]2+ | 1GlcN+2IdoA/GlcA+1 GlcNAc (4S) | 1054.4 | 1054.1 | ||
| C4 | 849.5(+2) | [M+6HXA+H]2+ | 4 | 2GlcN+2IdoA/GlcA (5S) | 1092.0 | 1092.0 |
| 889.7(+2) | [M+6HXA+2H]2+ | 2GlcN+2IdoA/GlcA (6S) | 1171.4 | 1172.0 | ||
| C5 | 1030.4(+2) | [M+8HXA]2+ | 5 | 3GlcN+2IdoA/GlcA (5S) | 1252.8 | 1253.1 |
| 1059.2(+2) | [M+8HXA]2+ | 1GlcN+3IdoA/GlcA+1 GlcNAc (5S) | 1310.4 | 1310.0 | ||
| C6 | 1030.5(+2) | [M+8HXA]2+ | 5 | 3GlcN+2IdoA/GlcA (5S) | 1253.0 | 1253.1 |
| C7 | 898.3(+2) | [M+4HXA+2H]2+ | 5 | 1GlcN+3IdoA/GlcA+1 GlcNAc (6S) | 1390.6 | 1390.0 |
| 940.7(+2) | [M+5HXA+2H]2+ | 5 | 2GlcN+2IdoA/GlcA+1GlcNAc (6S) | 1374.4 | 1375.0 | |
| 1058.4(+2) | [M+6HXA+2H]2+ | 6 | 3GlcN+3IdoA/GlcA (6S) | 1508.8 | 1509.1 | |
| C8 | 896.0(+2) | [M+2HXA+2H]2+ | 6 | 3GlcN+3IdoA/GlcA (7S) | 1588.0 | 1589.0 |
| 949.5 (+2) | [M+4HXA+2H]2+ | 5 | 3GlcN+2IdoA/GlcA (8S) | 1493.0 | 1492.9 | |
| 1061.2(+2) | [M+7HXA+2H]2+ | 5 | 3GlcN+2IdoA/GlcA (7S) | 1413.4 | 1413.0 | |
| 1148.5 (+2) | [M+7HXA+2H]2+ | 6 | 3GlcN+3IdoA/GlcA (7S) | 1588.0 | 1589.0 | |
| C9 | 949.7 (+2) | [M+4HXA+2H]2+ | 5 | 3GlcN+2IdoA/GlcA (8S) | 1493.4 | 1492.9 |
| 1239.8(+2) | [M+8HXA+2H]2+ | 6 | 3GlcN+3IdoA/GlcA (8S) | 1669.6 | 1669.0 | |
| C10 | 1027.9 (+3) | [M+10HXA+3H]3+ | 7 | 4GlcN+3IdoA/GlcA (11S) | 2070.0 | 2069.9 |
| 1068.0 (+3) | [M+12HXA+H]3+ | 7 | 4GlcN+3IdoA/GlcA (10S) | 1991.0 | 1990.0 | |
| 1126.7(+3) | [M+12HXA+H]3+ | 8 | 4GlcN+4IdoA/GlcA (10S) | 2167.1 | 2166.0 | |
| C11 | 1075.0(+4) | [M+17HXA]4+ | 9 | 4GlcN+5IdoA/GlcA (13S) | 2583.0 | 2581.9 |
| 1127.0(+4) | [M+18HXA]4+ | 9 | 4GlcN+4IdoA/GlcA+1GlcNAc (14S) | 2690.0 | 2688.9 | |
| 1111.5(+4) | [M+17HXA+2H]4+ | 9 | 5GlcN+4IdoA/GlcA (15S) | 2727.0 | 2726.8 | |
| C12 | 617.8(+7) | [M+16HXA+3H]7+ | 10 | 4GlcN+5IdoA/GlcA+1GlcNAc (12S) | 2705.6 | 2705.0 |
| 637.8(+7) | [M+17HXA+4H]7+ | 10 | 5GlcN+5IdoA/GlcA (13S) | 2743.6 | 2743.0 | |
| C13 | 532.9(+8) | [M+11HXA+8H]8+ | 11 | 6GlcN+5IdoA/GlcA (16S) | 3143.4 | 3143.9 |
| 763.9 (+6) | [M+15HXA+6H]6+ | 11 | 6GlcN+5IdoA/GlcA (15S) | 3062.4 | 3063.9 | |
| 723.2 (+7) | [M+18HXA+4H]7+ | 12 | 6GlcN+6IdoA/GlcA(15S) | 3240.4 | 3240.0 | |
| C14 | 723.3(+7) | [M+18HXA+4H]7+ | 12 | 6GlcN+6IdoA/GlcA(15S) | 3241.1 | 3240.0 |
| C15 | 618.1(+9) | [M+18HXA+9H]9+ | 13 | 7GlcN+6IdoA/GlcA(19S) | 3736.8 | 3735.8 |
| 618.1(+9) | [M+18HXA+9H]9+ | 14 | 7GlcN+7IdoA/GlcA(17S) | 3736.8 | 3737.0 | |
| 808.8(+7) | [M+18HXA+9H]9+ | 13 | 7GlcN+6IdoA/GlcA(19S) | 3836.6 | 3735.8 | |
| 808.8(+7) | [M+18HXA+9H]9+ | 14 | 7GlcN+7IdoA/GlcA(17S) | 3836.6 | 3737.0 | |
Major ion detected;
DP, Degree of polymerization;
1ΔUA+2GlcN+2IdoA/GlcA+1GlcNAc(5S): one residue of 4-deoxy-α-L-threo-hex-4-eno-pyranosyluronic acid, two residues of 2-deoxy-2-amino-D-glucopyranose, two residues consisting of either L-idopyranosyluronic acid or D-glucopyranosyluronic acid, one residue of 2-deoxy-2-acetamido-D-glucopyranose, and five sulfo groups
Acknowledgements
The authors are grateful for support by a Grant-in-Aid for Scientific Research (20590032), and Special Funds for Education and Research (Development of SPECT Probes for Pharmaceutical Innovation) from the Ministry of Education, Culture, Sports, Science and Technology, Japan (TT), and by the US National Institutes of Health in the form of grants GM38060, HL096972 and HL101721 (RJL).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Andreson LO, Barrowcliffe TW, Holmer E, Johnson EA, Sims GE. Anticoagulant properties of heparin fractionated by affinity chromatography on matrix-bound antithrombin III and by gel filtration. Thromb. Res. 1976;9:575–583. doi: 10.1016/0049-3848(76)90105-5. [DOI] [PubMed] [Google Scholar]
- 2.Harenberg J. Pharmacology of low molecular weight heparins. Semin. Thromb. Hemost. 1990;16:12–18. [PubMed] [Google Scholar]
- 3.Johnson EA, Mulloy B. The molecular weight range of commercial heparin preparations. Carbohydr. Res. 1976;51:119–127. doi: 10.1016/s0008-6215(00)84041-0. [DOI] [PubMed] [Google Scholar]
- 4.Linhardt RJ, Gunay NS. Production and chemical processing of low molecular weight heparins. Semin. Thromb. Hemost. 1999;25(Suppl. 3):5–16. [PubMed] [Google Scholar]
- 5.Gervin AS. Complications of heparin therapy. Surg. Gynecol.Obstet. 1975;40:789–796. [PubMed] [Google Scholar]
- 6.Freedman MD. Pharmacodynamics, clinical indications, and adverse effects of heparin. J. Clin. Pharmacol. 2004;32:584–596. doi: 10.1002/j.1552-4604.1992.tb05765.x. [DOI] [PubMed] [Google Scholar]
- 7.Hirsh J, Raschke R. Heparin and low-molecular-weight heparin. CHEST. 2004;126:188S–203S. doi: 10.1378/chest.126.3_suppl.188S. [DOI] [PubMed] [Google Scholar]
- 8.Linhardt RJ, Claude S. Hudson Award address in carbohydrate chemistry. Heparin: structure and activity. J. Med. Chem. 2003;46:2551–2554. doi: 10.1021/jm030176m. [DOI] [PubMed] [Google Scholar]
- 9.Green D, Hirsh J, Heit J, Prins M, Davidson B, Lensing AW. Low molecular weight heparin: a critical analysis of clinical trials. Phamacol. Rev. 1994;46:89–109. [PubMed] [Google Scholar]
- 10.Breddin HK, Fareed J, Bender N. Low molecular weight heparins. Haemostasis. 1988;18:1–87. doi: 10.1159/000215865. [DOI] [PubMed] [Google Scholar]
- 11.Shivley JE, Conrad HE. Formation of anhydrosugars in the chemical depolymerization of heparin. Biochemistry. 1976;15:3932–3942. doi: 10.1021/bi00663a005. [DOI] [PubMed] [Google Scholar]
- 12.Lindahl U, Backstrom GE, Thunberg JYL. Heparin fragments having selective anticoagulation activity. 1980 January 4; U.S. Patent 4,303,651.
- 13.Fussi F. Process for obtaining low molecular weight heparins endowed with elevated pharmacological properties and products so obtained. 1981 July 28; U. S. Patent 4, 281, 108.
- 14.Mardiguian J. Heparin esters and process for their preparation. 1984 April 3; U.S. Patent 4, 440, 926.
- 15.Uzan A. Sulfated polysaccharides obtained from heparin, preparation process, pharmaceutical composition and use thereof. 1995 June 6; U.S. Patent 5,849,721.
- 16.Bianchini P, Mascellani G. Novel oligosaccharides having pharmacological properties by depolymerization of heparin. 1988 December 13; U.S.Patent 4,791,195.
- 17.Bisio A, Guglieri S, Frigerio M, Torri G, Vismara E, Cornelli U, Bensi D, Gonella S, De Ambrosi L. Controlled γ-ray irradiation of heparin generates oligosaccharides enriched in highly sulfated sequences. Carbohydr. Polym. 2004;55:101–112. [Google Scholar]
- 18.Linhardt RJ, Grant A, Cooney CL, Langer R. Differential anticoagulant activity of heparin fragments prepared using microbial heparinase. J. Biol. Chem. 1982;257:7310–7313. [PubMed] [Google Scholar]
- 19.Nielsen JI. Process of using light absorption to control enzymatic depolymerization of heparin to produce low molecular weight heparin. 1992 April 21; U.S. Patent 5,106,734.
- 20.Linhardt RJ, Loganathan D, Al-Hakim A, Wang HM, Walenga JM, Hoppensteadt D, Fareed J. Oligosaccharide mapping of low molecular weight heparins: structure and activity differences. J Med Chem 1990. 1990;33:1639–1645. doi: 10.1021/jm00168a017. [DOI] [PubMed] [Google Scholar]
- 21.Fussi F. Process for obtaining low molecular weight heparins. 1982 European Patent GB2, 002, 406B.
- 22.Fussi F. Oligo-heteropolysaccharides a active semblable a celle de l'heparine, procede pour leur preparation et compositions therapeutiques correspondantes. 1978 French Patent #FR 78 23499.
- 23.Burana-osot J, Hosoyama S, Nagamoto Y, Suzuki S, Linhardt RJ, Toida T. Photolytic depolymerization of alginate. Carbohydr. Res. 2009;344:2023–2027. doi: 10.1016/j.carres.2009.06.027. [DOI] [PubMed] [Google Scholar]
- 24.Burana-osot J, Soo nthornchareonnon N, Hosoyama S, Linhardt RJ, Toida T. Partial depolymerization of pectin by a photochemical reaction. Carbohydr. Res. 2010;345:1205–1210. doi: 10.1016/j.carres.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 25.Higashi K, Ly M, Wang Z, Masuko S, Bhaskar U, Sterner E, Zhang F, Toida T, Dordick JS, Linhardt RJ. Controlled photochemical depolymerization of K5 heparosan, a bioengineered heparin precursor. Carbohydr. Polym. 2011;86:1365–1370. doi: 10.1016/j.carbpol.2011.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chaidedgumjorn A, Suzuki A, Toyoda H, Toida T, Imanari T, Linhardt RJ. Conductivity detection for molecular mass estimation of per-O-sulfonated glycosaminoglycans separated by high-performance size-exclusion chromatography. J. Chromatogr. A. 2002;959:95–102. doi: 10.1016/s0021-9673(02)00446-6. [DOI] [PubMed] [Google Scholar]
- 27.Edens RE, Al-Hakim A, Weiler JM, Rethwisch DG, Fareed J, Linhardt RJ. Gradient Polyacrylamide Gel Electrophoresis for Determination of the Molecular Weights of Heparin Preparations and Low-Molecular-Weight Heparin Derivatives. J. Pharm. Sci. 1992;81:823–827. doi: 10.1002/jps.2600810821. [DOI] [PubMed] [Google Scholar]
- 28.Honda S, Suzuki S. Common conditions for high-performance liquid chromatographic microdetermination of aldoses, hexosamines, and sialic acids in glycoproteins. Anal. Biochem. 1984;142:167–174. doi: 10.1016/0003-2697(84)90533-5. [DOI] [PubMed] [Google Scholar]
- 29.Loshe DL, Linhardt RJ. Purification and characterization of heparin lyases from Flavobacterium heparinum. J. Biol. Chem. 1992;267:24347–24355. [PubMed] [Google Scholar]
- 30.Linhardt RJ. Analysis of glycosaminoglycans with polysaccharide lyases. Curr. Meth. in Mole. Biol. 2001 doi: 10.1002/0471142727.mb1713bs48. UNIT 17.13B.1-17.13B.16. [DOI] [PubMed] [Google Scholar]
- 31.Solakyildirim K, Zhang Z, Linhardt RJ. Ultraperformance liquid chromatography with electrospray ion trap mass spectrometry for chondroitin disaccharide analysis. Anal. Biochem. 2010;397:24–28. doi: 10.1016/j.ab.2009.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blažková A, Brezová V, Soldánová Z, Stasko A, Soldan M, Čeppan M. Photocatalytic degradation of heparin over titanium dioxide. J. Mater. Sci. 1995;30:729–733. [Google Scholar]
- 33.Ohno T, Sarukawa K, Tokeida K, Matsumura M. Morphology of TiO2 photocatalyst (degussa, P-25) consisting of anatase and rutile crystalline phases. J. Catal. 2001;203:82–86. [Google Scholar]
- 34.Harbour JR, Tromp J, Hair ML. Photogeneration of hydrogen peroxide in aqueous TiO2 dispersions. Can. J. Chem. 1985;63:204–208. [Google Scholar]
- 35.Jaeger CD, Bard AJ. Spin trapping and electron spin resonance detection of radical intermediates in the photodecomposition of water at titanium dioxide particulate systems. J. Phys. Chem. 1979;83:3146–3152. [Google Scholar]
- 36.Noda H, Oikawa K, Kamada H. ESR Study of Active Oxygen Radicals from Photoexcited Semiconductors Using the Spin-Trapping Technique. Bull. Chem. Soc. Jpn. 1992;65:2505–2509. [Google Scholar]
- 37.Vismara E, Pierini M, Guglieri S, Liverani L, Mascellani G, Torri G. Structural modification induced in heparin by a Fenton-type depolymerization process. Semin. Thromb. Hemost. 2007;33:466–477. doi: 10.1055/s-2007-982077. [DOI] [PubMed] [Google Scholar]
- 38.Vismara E, Pierini M, Mascellani G, Liverani L, Lima M, Guerrini M, Torri G. Low-molecular-weight heparin from Cu2+ and Fe2+ fenton type depolymerization processes. Thromb. Haemost. 2010;103:613–622. doi: 10.1160/TH09-02-0084. [DOI] [PubMed] [Google Scholar]
- 39.Sudo M, Sato K, Chaidedgumijorn A, Toyoda H, Toida T, Imanari T. 1H nuclear magnetic resonance spectroscopic analysis for determination of glucuronic and iduronic acids in dermatan sulfate, heparin, and heparin sulfate. Anal. Biochem. 2001;297:42–51. doi: 10.1006/abio.2001.5296. [DOI] [PubMed] [Google Scholar]
- 40.Linker A, Meyer K, Hoffman P. The production of unsaturated uronides by bacterial hyaluronidases. J. Biol. Chem. 1955;219:13–25. [PubMed] [Google Scholar]
- 41.Masuko S, Higashi K, Wang Z, Bhaskar U, Hickey AM, Zhang F, Toida T, Dordick JS, Linhardt RJ. Ozonolysis of the double bond of the unsaturated uronate residue in low molecular weight heparin and K5 heparosan. Carbohydr. Res. 2011;346(13):1962–1966. doi: 10.1016/j.carres.2011.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thanawiroom C, Rice KG, Toida T, Linhardt RJ. Liquid chromatography/mass spectrometry sequencing approach for highly heparin-derived oligosaccharides. J. Biol. Chem. 2004;279:2608–2615. doi: 10.1074/jbc.M304772200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material to Higashi et al. “Photochemical preparation of a novel low molecular weight heparin”
Supplemental figure 1. Polyacrylamide gel electrophoresis of LMWHs.
Lanes contain: a. controlled heparinase depolymerization of bovine lung heparin; b. pLMWH; c, RD heparin; d, OP2123; e, Logiparin; and f. Enoxaparin. 5 μg of controlled heparinase depolymerization of bovine lung heparin (a) and 7.5 μg LMWHs (b-f) were separated by PAGE on a 22% acrylamide gel and stained with 0.5% (w/v) Alcian blue in 2% (v/v) acetic acid aqueous solution.