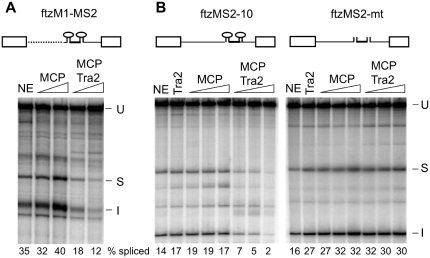
Figure 3.
Tethering of Tra2 to an intron is sufficient to initiate splicing repression. (A) Splicing of ftzM1-MS2, in which the ISS of ftzM1 (27) was replaced by RNA sequences from the bacteriophage MS2 (shown as stem loops) that bind MCP and MCP fusion proteins, are shown. Other intron sequences upstream of the polypyrimidine tract (dotted line) derive from the M1 intron. The exons and 3′-end of the intron derive from ftz. Splicing was carried out in the presence of S2 nuclear extract (NE) or extracts supplemented with 100 and 300 nM MCP or MCP–Tra2 fusion protein. The mobilities of in vitro splicing products and the percentage of splicing are indicated as in Figure 2. (B) Shown are splicing reactions carried out on substrates in which two wt MCP-binding sites (ftzMS2-10), or mutant sites (ftzMS2-mt) were inserted into the intron of the ftz gene. In contrast to ftzM1, these splicing substrates lack any tra2 RNA sequences. Nuclear extracts (NE) were supplemented with 500 nM Tra2 or with 100, 200 or 400 nM MCP or MCP-Tra2 protein. The positions of unspliced RNA (U), spliced product (S) and spliced intron (I) are indicated. The percentage of total signal from splice products and intermediates is indicated below each lane.