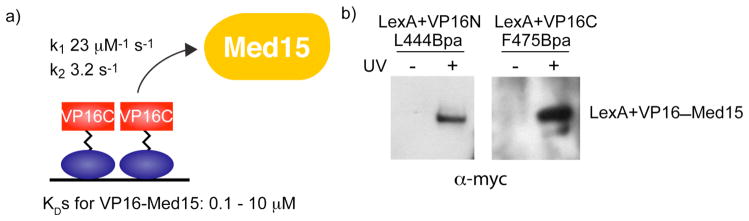
Figure 3.
In vivo photocrosslinking captures the moderate affinity interaction between LexA+VP16 and the Mediator protein, Med15. a) VP16 has been shown to interact transiently with the coactivator Med15, as determined by measured kinetic rate constants. Equilibrium binding measurements place the affinity of the TAD for Med15 in the moderate category, with DNA-bound homodimers exhibiting the highest affinity (0.1 μM) and isolated TADs in the low to mid-micromolar range (16, 39). (b) Live yeast cells bearing plasmids expressing LexA+VP16N L444Bpa or LexA+VP16C F475Bpa fusion proteins, in addition to a plasmid expressing myc-Med15(1–416) were irradiated with UV light (365 nm) for 30 minutes. Subsequently, cell lysates were immunoprecipitated with αLexA and analyzed by Western blot (αmyc). For both constructs, a crosslink with Med15 is observed. Supporting Figure S1 shows expression of myc-Med15(1–416) and Supporting Figure S2 shows the full Western blot, complete with molecular weight references.