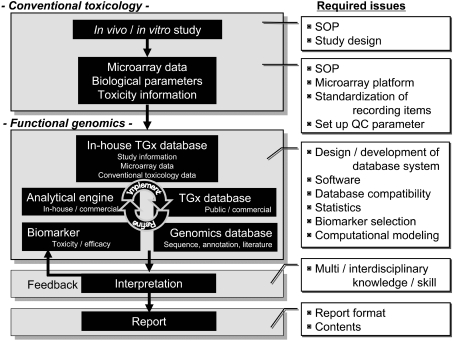
Fig. 1.
General flow of a TGx study. The general flow of a TGx study is presented. Conventional toxicologic parameters, such as body / organ weights, histopathological findings, blood chemistry and toxico / pharmacokinetics, and functional genomics information, such as microarray data, are collected. The genomics data sets are huge and need to be organized into a well-designed database. Interpretation of the genomics data depends on the quality of the database, and analytical tools and an experienced researchers’ interdisciplinary knowledge and skills in biology, toxicology, statistics and computational sciences. A number of issues are yet to be determined to establish a standard operating procedure (SOP) for the public, including the content / format of the final report, recording items, statistical analysis to be performed for genomics data, etc. All the information should be appropriately recorded so that the obtained TGx data can be exchangeable across laboratories.