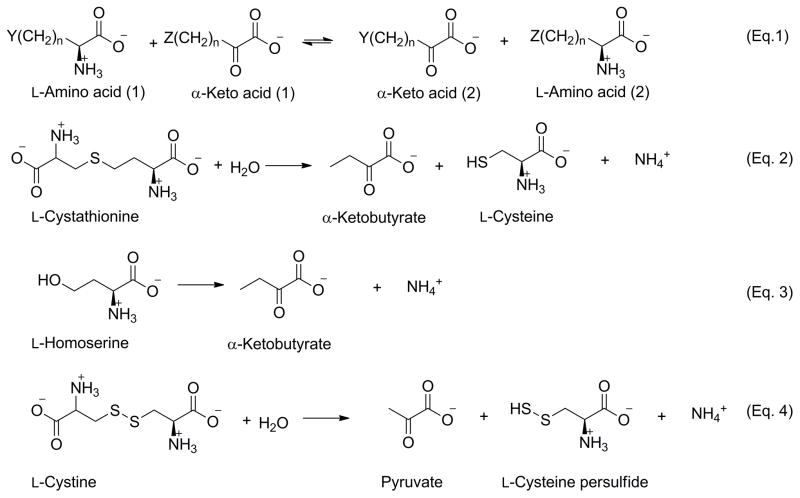
Fig. 3.
Specificity of glutamine transaminase K (GTK) and cystathionine γ-lyase. Transamination reactions between amino acids and α-keto acids catalyzed by GTK are depicted in equation 1, where n = 1 or 2, and Y and Z can range in size from H- (e.g. α-ketobutyrate, n = 2) to relatively large hydrophobic moieties such as CH3S-(KMB, n = 2; L-methionine, n = 2) or C6H5- (phenylpyruvate, n = 1; L-phenylalanine, n = 1). Cystine [Y or Z =-SSCH2CH(NH3+)CO2−, n = 1] and some other sulfur-containing amino acids, in which Y or Z possesses a terminal charged grouping, are also substrates. Cystathionine γ-lyase catalyzes several γ-elimination, β-elimination and replacement reactions. Some of the more important reactions that are pertinent to the current study are shown here. The enzyme catalyzes a γ-elimination reaction with L-cystathionine (equation 2) and with L-homoserine (equation 3). In both cases, the α-keto acid product is α-ketobutyrate. Cystathionine γ-lyase also catalyzes a β-elimination reaction with L-cystine (equation 4). In this case, the α-keto acid product is pyruvate.