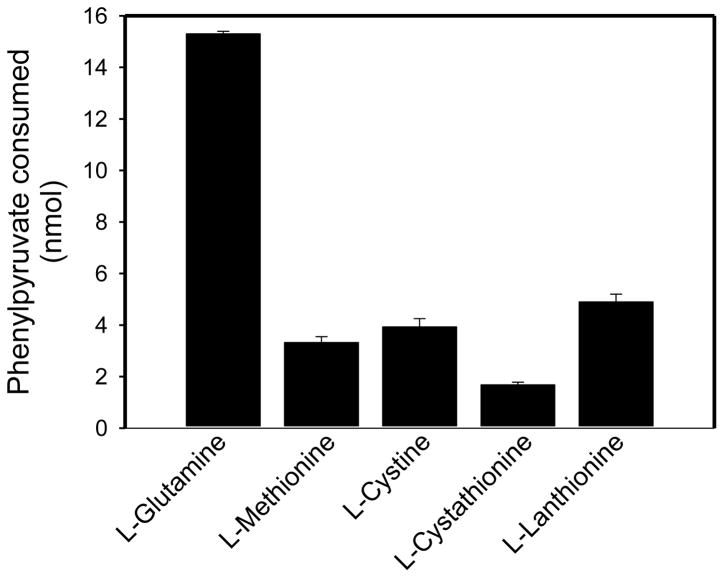
Fig. 5.
rhGTK-catalyzed transamination of phenylpyruvate with glutamine and various sulfur-containing amino acids. Except where indicated, the reaction mixture (50 μl) contained 1 mM amino acid, 0.4 mM phenylpyruvate, 100 mM potassium phosphate buffer (pH 7.4) and enzyme (2.15 mU). In the case of L-cystine the concentration was 0.4 mM and the buffer was 50 mM sodium pyrophosphate (pH 9.2). After incubation for 1 h at 37°C the amount of phenylpyruvate remaining in solution was determined relative to a blank reaction mixture lacking enzyme; n = 3.