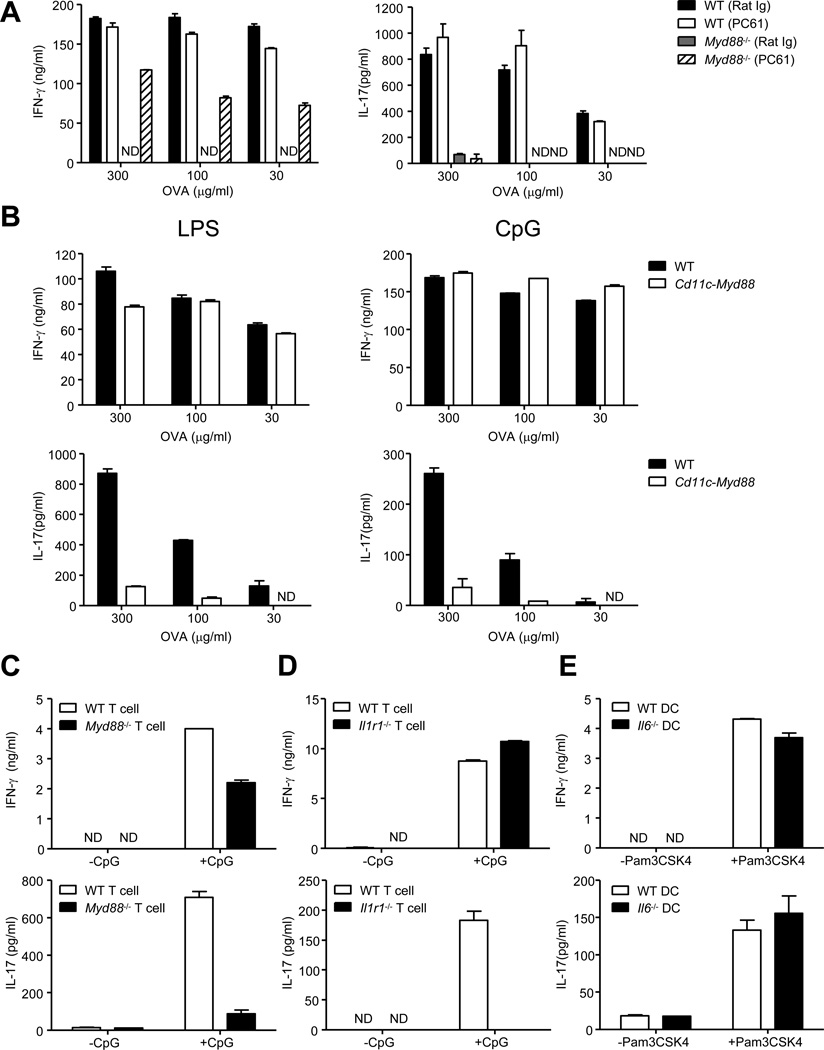
Figure 1. MyD88-dependent IL-1R signaling in T cells is required for Th17 cell priming and IL-6 is dispensable for Th17 cell priming in vitro.
(A) WT and Myd88−/− mice received rat anti-mouse CD25 antibody (PC61) or control antibody (Rat Ig) by intravenous route (25µg/mouse). Three days later, Treg cell depletion was confirmed (Figure S1A) and mice were immunized in the footpads (fp) with Ovalbumin (OVA) (50µg/fp) and LPS (5µg/fp) emulsified in incomplete Freund’s adjuvant (IFA). CD4+ T cells were purified from the draining lymph nodes at day 7 post-immunization and cultured with Tlr2−/−Tlr4−/− B cells as antigen presenting cells (APC)s in the presence of titrating doses of OVA for 72 hours. IFN-γ (left) and IL-17 (right) concentrations in the culture supernatants were determined by ELISA. (B) WT and Cd11c-Myd88 Tg mice were immunized in the fp with OVA (50µg/fp) and LPS (5µg/fp) (left panels) or OVA (50µg/fp) and CpG (5µg/fp) (right panels) emulsified in IFA. CD4+ T cells were activated to measure IFN-γ (upper panels) and IL-17 (lower panels) as described above. (C, D and E) Purified naïve CD4+ T cells (3×105) from the indicated strains were cultured in the presence of purified WT splenic DCs or Il6−/− splenic DCs (6×104) and anti-CD3 (10ng/ml) for 5 days in the presence or absence of the TLR ligands. Culture supernatants were collected and assayed for IFN-γ top panels) and IL-17 (bottom panels) by ELISA. Data are representative of three to four independent experiments. Bar graphs represent mean ± SEM.