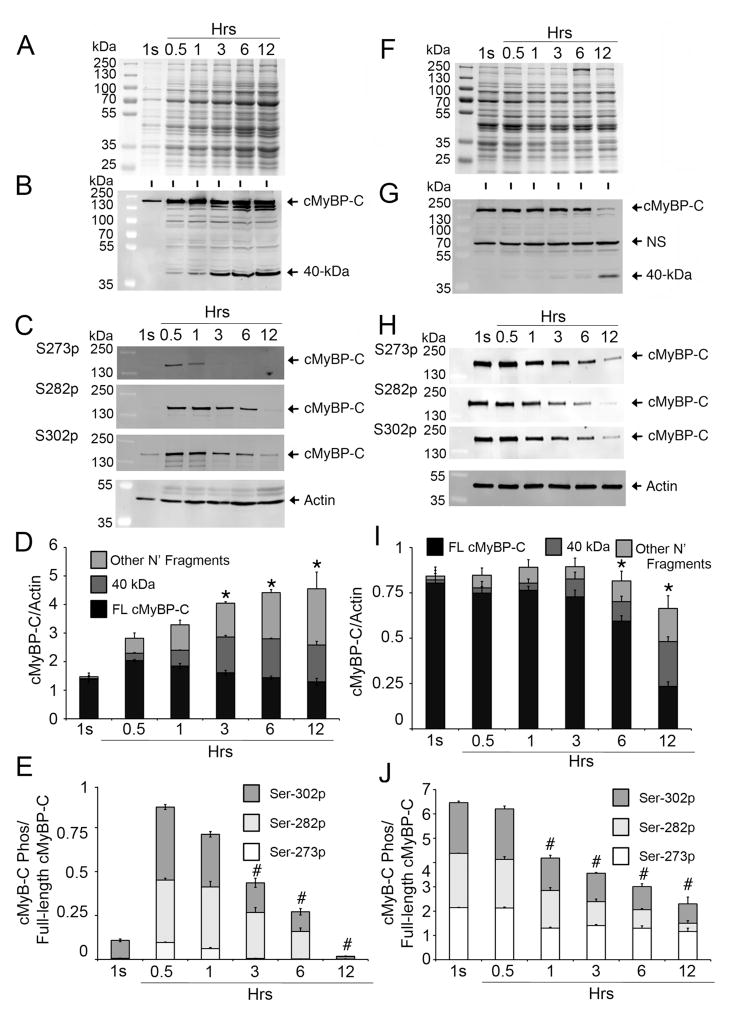
Fig 1. cMyBP-C is an easily releasable myofilament.
Rat LV tissue (100 mg) was incubated in 500 μl of PBS (effluent) at 37°C for 1 second (1s), and 0.5, 1, 3, 6 and 12 hrs. Effluent (10 μl) was used for SDS-PAGE (A), followed by Western blot analyses with rabbit anti-cMyBP-C2–14 antibody (B) and site-specific Ser-273 (S273p), Ser-282 (S282p) and Ser-302 (S302p) phospho antibodies (C). Sarcomeric α-actin was used as a loading control. Quantitative analyses (D) show the increase of full-length (FL) cMyBP-C, 40 kDa and other N′-fragments, either in total cMyBP-C or individually as FL, 40 kDa and other N′-fragments, over time (n=4, P<0.01 vs. 0.5 hr time point), but, at the same time, gradual reduction of cMyBP-C phosphorylation at Ser-273, Ser-282 and Ser-302 sites individually or in total (E, P<0.01 vs. 0.5 hr time point), suggesting an inverse relationship between the degradation and phosphorylation status of cMyBP-C. Ten μg of total protein from the tissue homogenates of the leftover rat heart tissue at different time points were resolved on SDS-PAGE (F) and analyzed by Western blot analyses with rabbit anti-cMyBP-C2–14 antibodies (G) and site-specific Ser-273 (S273p), Ser-282 (S282p) and Ser-302 (S302p) phospho antibodies (H). Quantitative analyses show (I) reduced total cMyBP-C with increased release of 40 kDa and other N′-fragments at 6- and 12-hr time points (n=4, P<0.01 vs. 1s time point). Phosphorylation status at the three sites was decreased in total or individually (J), starting from the 1-hr time point (n=4, P<0.01 vs. 1s time point). A nonspecific (NS) protein, which was not released into the effluent, was identified (Online Supplement Fig. 5).