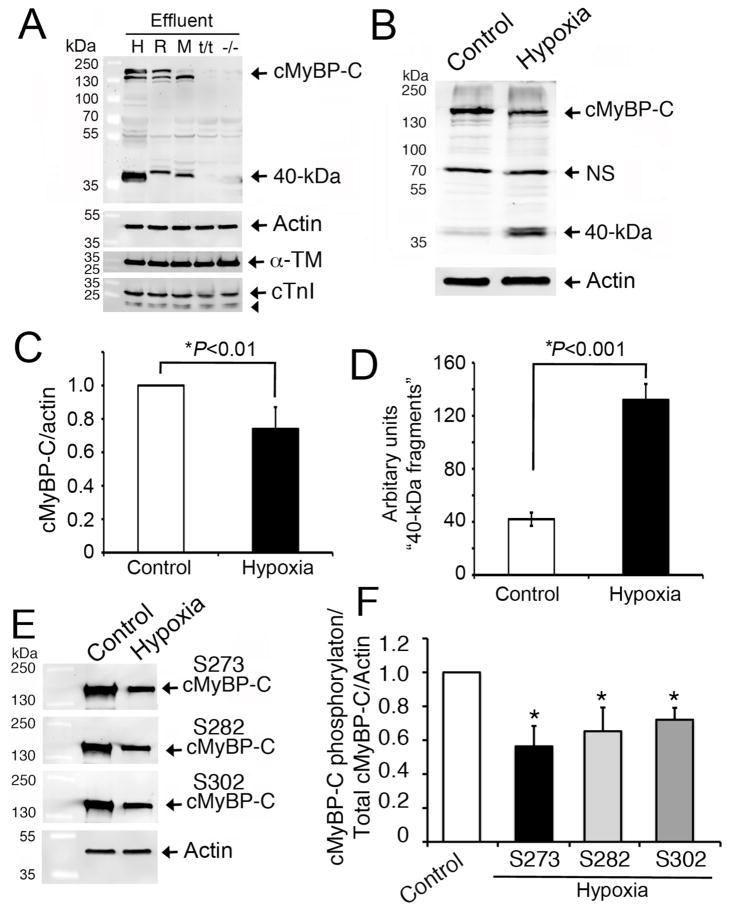
Fig 2. Release of the 40 kDa fragment is specific to cMyBP-C.
Representative Western blot analyses (n=3) show that the cleavage of 40 kDa N-terminal cMyBP-C fragments was consistent in human (H), rat (R) and mouse (M) heart effluents at 6 hrs and was absent in negative controls, such as cMyBP-C null (t/t) and systemic knockout (−/−) mouse hearts (A). LV tissue (100 mg) from the respective species was incubated in 500 μl of PBS (effluent) at 37°C for 6 hrs. Effluent (10 μl) was used for SDS-PAGE, followed by Western blot analyses with rabbit anti-cMyBP-C2–14 antibody. Sarcomeric α-actin, α-TM and cTnI (arrowhead shows its degraded fragments) were determined for controls. Three-day-old NRVMs exposed to hypoxia were compared to controls for cMyBP-C degradation. Representative Western blot analyses using 10 μg of total cell lysates with rabbit anti-cMyBP-C2–14 antibodies (B) show a significant decrease in full-length cMyBP-C (C) and an increase in 40 kDa fragments (D) post-hypoxia. Phosphorylation status of cMyBP-C at Ser-273, Ser-282 and Ser-302 was determined by Western blot analyses using site-specific antibodies (E). Data, which are normalized with full-length cMyBP-C and actin, are summarized for phosphorylation status (F). *P<0.01, hypoxia vs control (n=4).