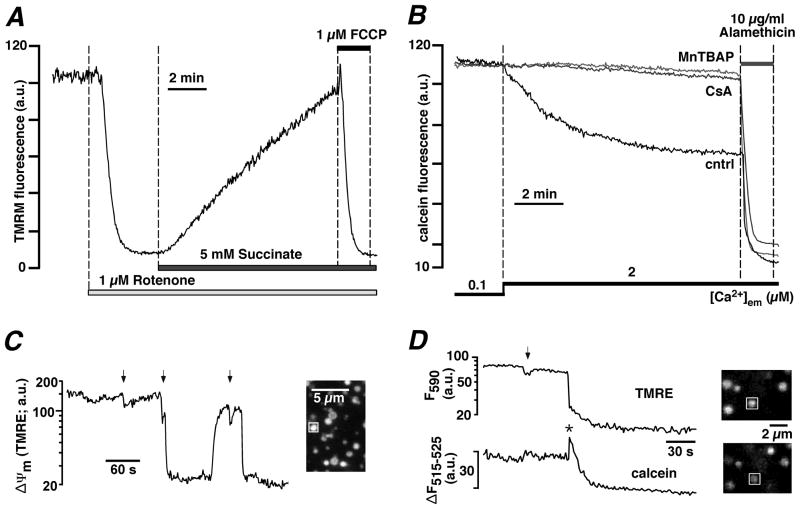
Figure 2. Measurements ofΔΨm and mPTP activity in permeabilized ventricular myocytes and isolated mitochondria.
A, ΔΨm monitored with the membrane potential sensitive dye TMRM in permeabilized rabbit ventricular myocytes upon inhibition of ETC complex I with rotenone and subsequent exposure to ETC complex II substrate succinate. The mitochondrial uncoupler FCCP completely depolarized Ψm. B, mPTP activity monitored with mitochondria entrapped calcein (620 Da) in permeabilized cat ventricular myocytes. Opening of mPTP was induced by enhanced ROS formation (prevented by superoxide and peroxynitrite scavenger MnTBAP, 50 μM) resulting from Ca2+-induced stimulation of mtNOS in the absence of L-arginine. Opening of mPTP resulted in the loss of calcein and a decrease of fluorescence. The pore forming antibiotic alamethicin induced maximum calcein release. Cyclosporine A (CsA, 5 μM) blocked mPTP opening. C, mPTP openings recorded from isolated mitochondria with TMRE. PTP opening can occur as brief incomplete (arrows) and prolonged maximal depolarizations. D, simultaneous monitoring of mPTP activity as changes in ΔΨm (TMRE) and loss of calcein in isolated mitochondria. Brief incomplete depolarizations (arrow) did not cause loss of calcein, however prolonged and complete depolarizations were accompanied by loss of calcein. The decline of calcein fluorescence was preceded by a transient increase (asterisk) caused by calcein dequenching. (Panel B: from Dedkova and Blatter, The Journal of Physiology, 2009, with permission; panels C and D: from Hüser, Rechenmacher and Blatter, Biophysical Journal, 1998, with permission).