Table 1. Summary of Favorable Interaction Types, Interaction Partners, and Geometry Definitionsa.
| interaction type | interacting atom types | cutoff distance, dcut [Å] | angle definitions |
|---|---|---|---|
| hydrogen bond | hdon | hacc | 0.2 | sp: 135.0 ≤ (hdon···hacc–X) ≤ 180.0b |
| sp2: 80.0 ≤ (hdon···hacc–X) ≤ 180.0b and | |||
30.0 ≤ (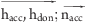 ) ≤ 90.0b ) ≤ 90.0b
|
|||
| sp3: 70.0 ≤ (hdon···hacc–X) ≤ 180.0b | |||
| metal | met | hacc | 0.2 | see hydrogen bond, with hdon replaced by met |
| ionic | cat | ani | 1.0 | |
| cation–dipole | cat | dneg | 0.7 | 120.0 ≤ (cat···dneg–X) ≤ 180.0 |
| cation-π | cat | π | 0.5 | 0.0 ≤ (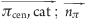 ) ≤ 45.0 ) ≤ 45.0 |
| dipolar | dpos | dneg | 0.4 | 60.0 ≤ (dneg1···dpos2–dneg2) ≤ 120.0 or |
| 150.0 ≤ (dneg1···dpos2–dneg2) ≤ 180.0b | |||
| halogen bond | σpos | σneg | 0.2 | 120.0 ≤ (σneg···σpos–X) ≤ 180.0 |
| 80.0 ≤ (σpos···σneg–X) ≤ 180.0 | |||
| hydrogen bond donor−π | hdon | π | 0.2 | 0.0 ≤ (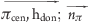 ) ≤ 45.0 ) ≤ 45.0 |
| see also hydrogen bond, with hacc replaced by πcen | |||
| π–π | π | π | 0.5 | (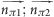 ) ∈ [0.0–35.0; 55.0–125.0; 145.0–180.0] ) ∈ [0.0–35.0; 55.0–125.0; 145.0–180.0] |
| parallel: distance (π1···π2cen) ≥ 2.0 Å and distance (π2···π1cen) ≥ 2.0 Å | |||
| orthogonal: distance (π1···π2cen) ≥ 2.0 Å or distance (π2···π1cen) ≥ 2.0 Å | |||
| vdW | hyd | hyd | 0.5 |
An interaction between two atoms A and B is counted as favorable if (a) their distance is below rvdW,A + rvdW,B + dcut, where rvdW are the van der Waals radii according to Bondi(34) and dcut is an interaction type-specific distance cutoff, and (b) all involved angular thresholds are fulfilled. X denotes a covalently attached non-hydrogen atom and n⃗ stands for the normal vector of the plane. For hydrogen bonds and metal interactions, angle definitions are dependent on the hybridization states of donor and acceptor, respectively.
Analogous terms with exchanged atom types are additionally used.
