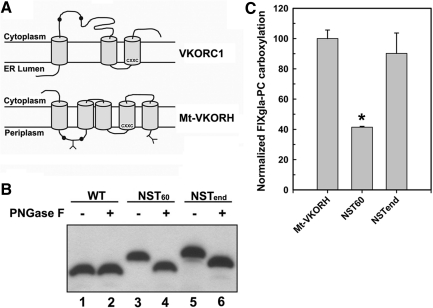
FIG. 6.
Localization with respect to the ER membrane of the conserved loop cysteines 57 and 65 and the C-terminus of Mt-VKORH by N-linked glycosylation mapping. (A) Schematic representation of the proposed membrane topology of human VKORC1 (32) and Mt-VKORH (35). Conserved loop cysteines are indicated by black dots and Y indicates the introduced N-linked glycosylation site. The CXXC redox center is located near the N-terminus of the third TMD in human VKORC1 and the fourth TMD in Mt-VKORH; both face the ER lumen. (B) Localization of the introduced glycosylation site in Mt-VKORH by Western blot assay. HPC4-tagged Mt-VKORH (WT) and its glycosylation mutants (NST60: glycosylation site was introduced between cysteine 57 and 65; NSTend: glycosylation site was introduced at the C-terminus) were transiently expressed in HEK293 cells. Cell lysate was treated with or without PNGase F before being subjected to SDS-NuPAGE. HPC4-tagged protein bands were probed with anti-HPC4 monoclonal antibody and visualized by ECL Western blot reagents. (C) Cell-based activity assay of Mt-VKORH glycosylation mutants. Mt-VKORH and its glycosylation mutants were transiently expressed in FIXgla-PC/HEK293 cells and the enzymatic activity was determined as described in the legend of Figure 1. Data are presented as mean±SD (n=3). *p<0.001, compared with wild-type Mt-VKORH. ER, endoplasmic reticulum.