Abstract
Background
Granulysin produced by cytolytic T cells directly contributes to immune defense against tuberculosis (TB). We investigated granulysin as a candidate immune marker for childhood and adolescent TB.
Methods
Peripheral blood mononuclear cells (PBMC) from children and adolescents (1–17 years) with active TB, latent TB infection (LTBI), nontuberculous mycobacteria (NTM) infection and from uninfected controls were isolated and restimulated in a 7-day restimulation assay. Intracellular staining was then performed to analyze antigen-specific induction of activation markers and cytotoxic proteins, notably, granulysin in CD4+ CD45RO+ memory T cells.
Results
CD4+ CD45RO+ T cells co-expressing granulysin with specificity for Mycobacterium tuberculosis (Mtb) were present in high frequency in TB-experienced children and adolescents. Proliferating memory T cells (CFSElowCD4+CD45RO+) were identified as main source of granulysin and these cells expressed both central and effector memory phenotype. PBMC from study participants after TB drug therapy revealed that granulysin-expressing CD4+ T cells are long-lived, and express several activation and cytotoxicity markers with a proportion of cells being interferon-gamma-positive. In addition, granulysin-expressing T cell lines showed cytolytic activity against Mtb-infected target cells.
Conclusions
Our data suggest granulysin expression by CD4+ memory T cells as candidate immune marker for TB infection, notably, in childhood and adolescence.
Introduction
Tuberculosis (TB) remains a leading cause of childhood mortality worldwide with 300,000 cases per year estimated globally and an unequal preponderance in developing countries [1], [2], [3]. While intense research efforts have focused on TB in adults, childhood TB has been largely neglected. Young children are at a much higher risk of progressing to active disease than adults. This is combined with higher mortality, with the highest prevalence among children under 2 years of age and the lowest between 5 and 10 years [4], [5]. Children also have a higher risk of extrapulmonary disease [6]. Due to its paucibacillary nature, diagnosis of childhood TB remains challenging. Most children with active TB are sputum smear negative. Less than 10–15% of children with proven TB exhibit sputum smear positivity for acid-fast bacteria [7]. The Mantoux tuberculin skin test (TST) in children also results in poor specificity, especially in countries where BCG vaccination is performed at birth. T cell-based interferon-gamma (IFNγ) release assays (IGRAs) offer some advantages [8], [9], [10]. The most deterministic factors of possible Mycobacterium tuberculosis (Mtb) infection in children, include compatible clinical signs and symptoms, an X-ray indicative of TB and likelihood of infection due to known contact [11].
Mtb primarily resides in phagosomes within macrophages and hence its antigens are presented by class II major histocompatibility complex (MHC) molecules to CD4+ T cells. Studies in mice lacking CD4+ T cells have demonstrated the importance of this T cell subset in the control of TB [12], [13], [14]. CD4+ T cells activated by Mtb antigens may also become cytotoxic T lymphocytes (CTL) [15], [16], [17], [18], [19]. The critical role of CD4+ T cells in control of Mtb is further underlined by the high incidence of TB in HIV+ individuals whose CD4+ T cell compartment is affected [20]. CD4+ CTL express granzymes, FAS ligand (Fas-L), perforin and granulysin [21]. Granzymes and perforin directly kill target cells, whereas Fas-L induces apoptosis in target cells [18]. Granulysin expresses potent bactericidal activity and can directly attack Mtb in combination with granzymes and perforin [22], [23], [24]. Granulysin is expressed after 3–5 days following T cell activation [25]. It is generally present in human CTL, but a homologous molecule has not been described in mice [26]. We determined the expression of the cytotoxic granule protein granulysin in Mtb-specific CD4+ T cells after 7 days of in vitro stimulation. Thus, detection of granulysin-expressing CD4+ memory T cells could serve as basis for development of an immune marker for diagnosis of childhood and adolescent TB.
Methods
Ethics statement
The study using samples from children [EA2/0128/4] was approved by the Charité Ethics Committee University Hospital Berlin, Germany. T cells lines were generated from PBMC from adult TST+ donors and approved by the Charité Ethics Committee University Hospital Berlin, Germany [EA1/200/08]. Written informed consent was provided by all study participants or their legal guardians. Study participant groups are listed in Table 1.
Table 1. Characteristics of the study population.
| Participants | LTBI | LTBI after therapy | Active TB patients | TB patients after therapy | NTM-infected children/adolescents | Healthy controls |
| Total number | 9 | 16 | 7 | 9 | 5 | 13 |
| Male | 5 | 8 | 3 | 6 | 4 | 7 |
| Female | 4 | 8 | 4 | 3 | 1 | 6 |
| Average range, years (median) | 2–17 (13) | 1–15 (10) | 1–16 (14) | 1–16 (6) | 2–13 (7) | 1–15 (9) |
Tablenotes: Latent TB infection LTBI; nontuberculous mycobacteria NTM; tuberculosis TB.
Study participants
To investigate the role of cytolytic T cells in childhood/adolescent TB, a total of 59 individuals (1–17 years; median age: 10 years) were recruited at the Department of Paediatric Pneumology and Immunology of the Charité University Hospital, Berlin, Germany. Individuals were characterized and defined as follows: Study participants with active TB had proven family contact to infectious TB and/or bacteriology (staining and/or culture and/or PCR), also positive TST and IGRA testing, as well as typical chest X-ray findings. Individuals with latent TB infection (LTBI) had proven family contact to patients with infectious TB and were also positive for TST and IGRA testing, but showed negative bacteriology and normal chest X-ray. Nontuberculous mycobacteria (NTM)-diseased participants showed cervical lymphadenitis, TST+ and negative IGRA. Bacterial culture for NTM after lymph node excision showed exclusive infection with M. avium and histological proof of epitheloid cell granulomatosis. In all cases, a TST was considered positive when induration was >5 mm. Quantiferon® TB-Gold In-Tube was used as IGRA according to manufacturer's instructions. An IGRA result was considered positive, when reaction to TB antigens minus the nil tube control was ≥0.35 IU/ml. Individuals with bacteriologically proven TB under antimycobacterial therapy were initially treated with a combination of isoniazid (INH), rifampicin (RMP) and pyrazinamide (PZA) [27]. For participants with advanced forms of TB, ethambutol (EMB) was included in initial treatment [28], [29]. All specimens showed full drug sensitivity. After termination of therapy, individuals underwent X-ray re-examination and bacteriological sputum negativity was confirmed. In the case of LTBI, chemoprophylaxis with INH was given for 9 months [30]. Controls were non-BCG-vaccinated individuals admitted to the hospital for non-TB-related diseases.
Restimulation assay
The 7-day restimulation assay has been described previously [31]. In brief, peripheral blood mononuclear cells (PBMC) were isolated by density centrifugation (Biocoll, Biochrom) in accordance with manufacturer's instructions. PBMC (2×105) were cultured in 200 µl RPMI 1640 medium (GIBCO, Invitrogen) containing 7.5% human serum (Sigma-Aldrich), 100 U/ml penicillin, 100 µg/ml streptomycin, 1 mM L-glutamine and 10 mM HEPES (all PAA laboratories) in 96-well round-bottom plates (NUNC). PBMC were stimulated with purified protein derivative (PPD) from Mtb (10 µg/ml) (Statens Serum Institute), recombinant Mtb fusion protein ESAT6-CFP10 (5 µg/ml) (courtesy of T.H. Ottenhoff/Leiden University) [32], recombinant EBNA1 protein from Epstein-Barr virus (EBV, 5 µg/ml), recombinant p65 protein from human cytomegalo-virus (HCMV, 5 µg/ml) or recombinant chimeric Chagas multiantigen from Trypanosoma cruzi (TcF, 5 µg/ml) (all Prospec). All stimuli were added at the beginning of culture and cells were kept at 37°C and 5% CO2. Cells were re-stimulated on day 6 with the same concentration of antigens, and Staphylococcal enterotoxin B from Staphylococcus aureus (SEB - Sigma-Aldrich) was also included as positive control for cytokine secretion (1 µg/ml). Brefeldin A (10 µg/ml) (Sigma-Aldrich) was added 4 h later and cells were cultured for an additional 12 h.
Intracellular cytokine staining (ICS)
After the 7-day stimulation period, cells were fixed and permeabilized using BD cytofix/cytoperm™ (BD Biosciences) according to manufacturer's instructions. Cells were then washed twice in BD perm/wash™ solution (BD Biosciences), and incubated at 4°C for 45 min with fluorescence-conjugated antibodies directed against surface and intracellular proteins. The following antibodies were used: anti-CD4 APC-Cy7, anti-CD8 PerCP-Cy5.5, anti-CD45RO PE-Cy7, anti-CD45RA PE-Cy7, anti-CCR7 APC, anti-GranzymeB Alexa700, anti-HLA-DR APC, anti-CD107a/b FITC, anti-CD40L APC, anti-CTLA-4 APC (all BD Biosciences); anti-IFNγ Pacific Blue, anti-granulysin PE, anti-perforin FITC (all eBioscience); anti-CCR5 Alexa647 (AbD Serotec); anti-CXCR3 FITC (BioLegend). After staining, cells were washed twice in BD perm/wash™ solution and resuspended in PBS containing 5% FCS. Measurements were performed using the LSRII flow cytometer (BD Biosciences) and data was analyzed using FACS-Diva software 6.1 (both BD Biosciences) and FlowJo version 8.8.4 (Treestar).
Proliferation assays
To measure the proliferation of T cells, PBMC were stained using CellTrace™ CFSE Cell Proliferation Kit (Invitrogen) according to manufacturer's instructions. Afterwards PBMC were plated and stimulated as described above.
Generation of granulysin-expressing T cell lines
PBMC from adult TST+ donors were obtained and several wells were stimulated with PPD using the 7-day restimulation assay described above. After stimulation, cells were pooled and stained with CD3 APC, CD4 APC-Cy7, CD8 PerCp-Cy5.5, CD25 PE-Cy7, CXCR3 FITC (all BD Bioscience). Cells showing a CD3+, CD4+, CD8−, CD25+, CXCR3+ phenotype were sorted using a BD FACS ARIA 2 (Becton Dickinson). A limiting dilution of 0.3 and 0.5 cells per well in a 96-well plate (Nunc) was performed and cells were cocultured with 1×105 autologous irradiated PBMC (28 grays). PPD (10 µg/ml) and recombinant IL-2 (40 U/ml) (Active Bioscience) were added in the first round of stimulation. Wells that showed visible growth were further expanded with irradiated PBMC, PHA (2.5 µg/ml) (Invitrogen) and IL-2 (40 U/ml).
Assessment of anti-mycobacterial activity of T cell lines
Autologous PBMC were isolated as described above. Monocytes were then isolated using a Monocyte Isolation Kit II (Miltenyi) and 1×104 monoyctes were plated per well in 96-well round-bottom plate in RPMI 1640 medium containing 7.5% human serum, 1 mM L-glutamine and 10 mM HEPES. Monocytes were infected with Mtb H37Rv at a multiplicity of infection (MOI) of 5 at 37°C and 5% CO2 for 24 h. After washing, different T cell lines were added at an effector∶target ratio of 10∶1. Plates were incubated for an additional 24 h at 37°C and 5% CO2. Afterwards cells were lysed with 100 µl of 0.1% Triton-X100 in PBS. Fifty µl of different dilutions of lysed cell suspension (1∶100; 1∶1,000; 1∶10,000; 1∶100,000) were plated on 7H11 agar plates. Plates were incubated at 37°C and colony forming units (CFU) were counted after 4 weeks.
Data analysis
Statistical analyses were performed using GraphPad Prism© V.5a software. For analysis of the CD4+ CD45RO+ T cell compartment and granulysin expression, study groups were compared using the Mann-Whitney U test. The same test was used for the comparison of T cell lines lacking or expressing granulysin. Changes in median fluorescence intensity of the different immune markers, between granulysin-negative and granulysin-expressing CD4+ CD45RO+ T cells, and in memory phenotype of granulysin-expressing T cells, were compared using the Wilcoxon signed-rank test.
Results
Antigen-specific induction of granulysin-expressing CD4+ CD45RO+ T cells
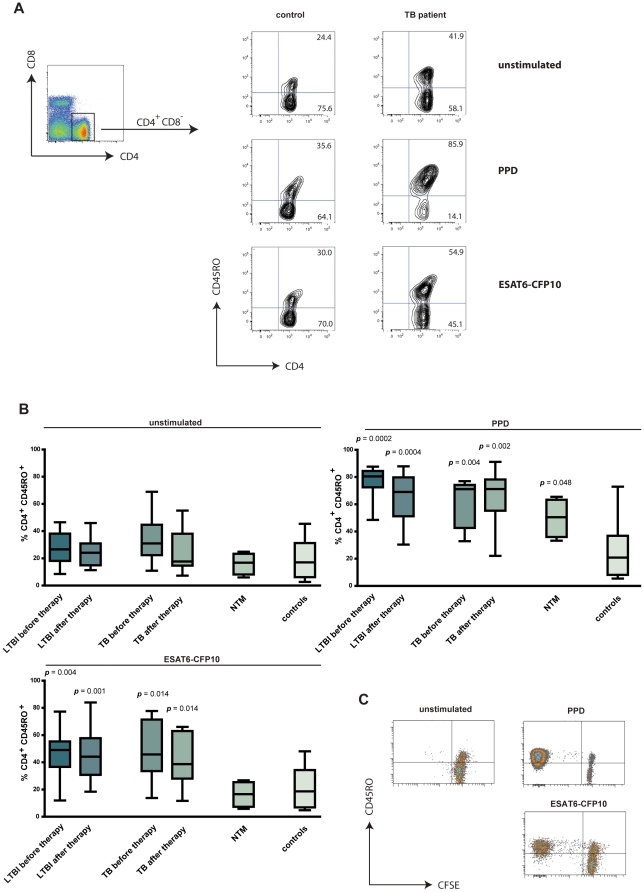
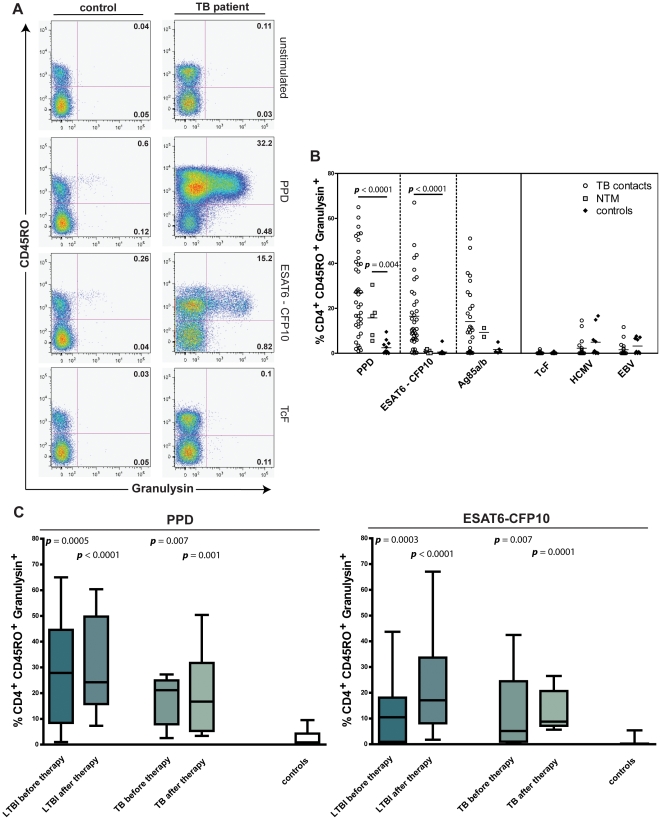
TB-experienced children and adolescents, before or after treatment, presented higher frequency of CD4+ CD45RO+ T cells after 7-day restimulation with Mtb antigens when compared to controls (Figure 1A). The frequency of CD4+ CD45RO+ T cells in individuals infected with NTM and in controls was not changed after stimulation with the recombinant fusion protein ESAT6-CFP10 (Figure 1B). These two proteins are absent from many environmental mycobacteria as well as from BCG.. Proliferation experiments revealed expansion of CD4+ CD45RO+ T cells after stimulation with PPD or ESAT6-CFP10 (Figure 1C) and not with control antigens (data not shown). This indicates enlargement of the memory T cell pool rather than recruitment of naive T cells during stimulation. On the contrary, CD8+ CD45RO+ memory cells did not proliferate during 7-day restimulation and a significant reduction in CD8+ frequencies after antigen-specific stimulation was observed (data not shown). Next, we investigated the induction of CTL activation in the CD4+ CD45RO+ T cell compartment by determining granulysin expression. The gating strategy and expression of granulysin after antigen-specific stimulation of CD4+ T cells in a representative TB patient and control sample are displayed in Figure 2A. Participants with TB – regardless of drug therapy – were compared to those with NTM infection and to controls (Figure 2B). A significant induction of granulysin expression in antigen-specific memory T cells was detected for both TB patients (p<0.001) and NTM-infected individuals (p<0.01), but was absent in controls. Granulysin-expressing T cells were reduced after stimulation with ESAT6-CFP10 in Mtb-experienced individuals only (p<0.001). Stimulation with Ag85, a protein shared by Mtb, BCG and NTM, did not reveal significant differences between the three groups. Mtb-unrelated antigens (TcF, HCMV, EBV) were also included for further verification of antigen specificity. Stimulation with TcF did not result in granulysin expression. In some TB patients as well as in some controls, granulysin induction was observed after stimulation with EBV and HCMV antigen, however, at low frequency. Next, participants with Mtb infection were classified according to clinical status and anti-TB therapy (Figure 2C). Both individuals with active Mtb infection and LTBI showed a significant increase in granulysin-expressing memory T cells after PPD and ESAT6-CFP10 restimulation. A similar induction was detected in participants that underwent TB drug therapy.
Figure 1. Increase of the CD4+ CD45RO+ memory T cell population after Mtb antigen stimulation.
A. Representative density plot showing gating strategy for CD4 and CD8 lymphocytes (left). CD4+ T cells were further gated for CD45ROlow versus high expression after: no stimulation (unstimulated), restimulation with purified protein derivative (PPD) or with ESAT6-CFP10. Representative examples for control (left) and active tuberculosis (TB) (right) are shown. B. Percentage of CD4+ CD45RO+ memory T cells unstimulated (left), after PPD (right) or ESAT6-CFP10 (bottom left) restimulation of 9 latent TB infection (LTBI) before therapy (light grey bar), 12 LTBI after drug therapy (dark grey bar), 7 active TB before drug therapy (light grey bar), 8 active TB after drug therapy (dark grey bar), 4 nontuberculous mycobacteria (NTM) (grey bar) and 13 controls without mycobacterial infection (white bar). Median represented by horizontal line, interquartile range by box, and range by whiskers. Significant difference of groups, in comparison to controls, is indicated (Mann-Whitney U test). C. Representative carboxyfluorescein diacetate succinimidyl ester (CFSE) proliferation dot plot of CD4+ T cells either unstimulated or after restimulation with PPD and ESAT6-CFP10, respectively.
Figure 2. Frequency of CD4+ CD45RO+ granulysin+ memory T cells after Mtb antigen stimulation.
A. Representative pseudo-color plot for CD45RO and granulysin expression by CD4+ T cells from control (left) and active tuberculosis (TB) (right) after 7-day restimulation. Peripheral blood mononuclear cells (PBMC) were unstimulated, stimulated with purified protein derivative (PPD), ESAT6-CFP10, or recombinant Trypanosoma cruzi antigen (TcF). B. Granulysin expression depicted for each antigen as percentage of CD4+ CD45RO+ memory T cells. Frequencies of 38 TB-experienced individuals (TB contacts) [latent TB infection (LTBI) and active TB before and after drug therapy] (circles), 5 nontuberculous mycobacteria (NTM) (squares), 12 controls without mycoabacterial infection (diamonds) are shown. PPD, ESAT6-CFP10, Ag85A/B, TcF, recombinant human cytomegalo-virus (HCMV) antigen Pp65, and recombinant Epstein-Barr virus (EBV) antigen EBNA-1, were used as antigens. C. Percentage of granulysin-expressing CD4+ CD45RO+ memory T cells from TB-experienced individuals: 10 LTBI before therapy (light grey bar), 12 LTBI after drug therapy (dark grey bar), 5 active TB (light grey bar), 9 active TB after drug therapy (dark grey bar) and 12 controls (white bar). Individual background values of unstimulated controls were subtracted. Median represented by horizontal line, interquartile range by box, and range by whiskers. Significant difference of groups, in comparison to controls, is indicated (Mann-Whitney U test).
Since CD4+CD45RO+ memory T cells contributed to the vast majority of granulysin-expressing cells after antigen restimulation (Figure 2A), we interrogated whether granulysin+ cells were derived from proliferating antigen-specific CD45RO+ memory T cells (Figure S1). Stimulation with PPD and ESAT6-CFP10 caused marked proliferation of CD45RO+ memory T cells, which were the main source of granulysin within the CD4+ T cell compartment. In contrast, the mitogen PHA induced strong T cell proliferation but almost no granulysin induction.
Granulysin-expressing T cells reveal central and effector memory phenotype, are long-lived and coexpress IFNγ
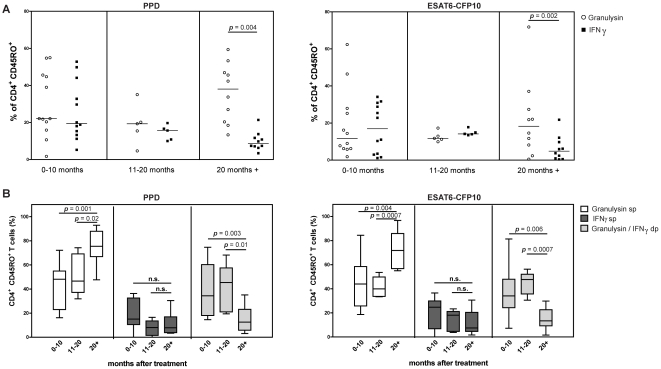
Further analysis revealed that after restimulation with ESAT6-CFP10, the majority of granulysin-expressing T cells presented an effector memory phenotype (CD45RA− CCR7−) (p<0.01) (Figure S2). The same trend was observed after PPD restimulation, but results did not reach statistical significance. We next determined the longevity of this memory response after completion of TB drug therapy. To address this, we analyzed frequencies of antigen-induced granulysin- and IFNγ-expressing CD4+ CD45RO+ T cells in children recruited at different time points after TB drug therapy (Figure 3A). During the first 20 months after completion of drug therapy, both IFNγ and granulysin were observed. Children, who had completed TB drug therapy more than 20 months earlier, continued to show elevated frequencies of granulysin-expressing CD4+ CD45RO+ T cells, which were significantly higher compared to IFNγ (p<0.01). Hence, granulysin-expressing CD4+ T cells specific for Mtb are long-lived. The coexpression of granulysin and IFNγ was analyzed more precisely in the three different study groups (Figure 3B). Frequencies of IFNγ-producing single-positive memory T cells remained stable over time, whereas the proportion of IFNγ and granulysin double-positive memory T cells declined. However, the fraction of granulysin single-positive memory T cells increased markedly over time. These data suggest a shift over time from IFNγ-/granulysin double-positive memory T cells towards long-lived granulysin single-positive T cells after completion of drug therapy.
Figure 3. Antigen-specific induction of granulysin and/or IFNγ in CD45RO+ memory T cells of TB patients at different time points after drug therapy.
A. Expression of granulysin (white circles) and interferon-gamma (IFNγ) (black squares) of CD4+ CD45RO+ memory T cells in peripheral blood mononuclear cells (PBMC) stimulated with purified protein derivative (PPD) (left) or recombinant ESAT6-CFP10 (right). Individual background values of unstimulated controls were subtracted. Medians for each group and statistical significance are indicated (Wilcoxon signed-rank test). B. Percentage of CD4+ CD45RO+ memory T cells expressing granulysin alone [granulysin single-positive (sp); white bars], IFNγ alone (IFNγ sp; grey bars) or both [granulysin/IFNγ double-positive (dp); light grey bars] after stimulation with PPD (left) or recombinant ESAT6-CFP10 (right). Median represented by horizontal line, interquartile range by box, and range by whiskers. Significance between different groups indicated (Mann-Whitney U test).
Coexpression of surface markers and additional CTL effector molecules by granulysin-expressing CD4+ T cells
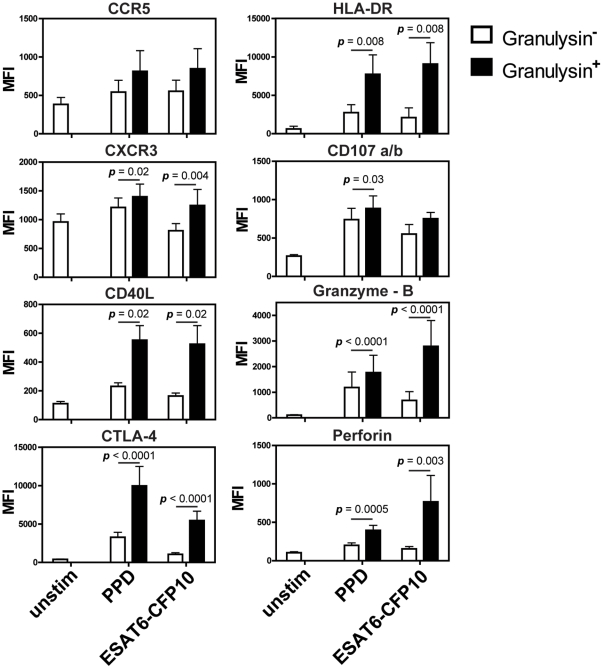
Memory T cells are characterized by the expression of distinct sets of chemokine receptors. We analyzed expression of the chemokine receptors, CCR5 and CXCR3, characteristic for type 1 helper T cells [33], [34], as well as activation markers (HLA-DR, CTLA-4, CD40L) [35], [36], [37] and additional cytotoxic markers (granzyme B and perforin) in granulysin-expressing T cells upon antigen stimulation (Figure 4). CXCR3 was significantly elevated on granulysin+ T cells, whereas CCR5 expression remained stable. Antigen-specific CD4+ CD45RO+ T cells also surface-expressed activation markers CD40L, CTLA-4 and HLA-DR at elevated intensity. Moreover, the cytotoxic proteins granzyme-B and perforin were highly induced in granulysin-expressing CD4+ T cells (Figure 4).
Figure 4. Median fluorescence intensity of different immune markers in granulysin-expressing CD4+ CD45RO+ memory T cells.
PBMC (2×105) from active TB and LTBI were stimulated with purified protein derivative (PPD), recombinant ESAT6-CFP10 or left unstimulated (unstim). Median fluorescence intensity (MFI) was measured in CD4+ CD45RO+ memory T cells and compared to T cells expressing granulysin upon restimulation (black bar) versus granulysin-negative T cells (white bar). Background expression is shown for unstimulated controls. Different numbers of patient samples were included in the analysis (CCR5 n = 9; CXCR3 n = 10; CD40L n = 7; CTLA-4 n = 15; HLA-DR n = 8; granzyme-B n = 21; perforin n = 12). Bars indicate arithmetic mean and overlying error bars represent standard error. Significant differences between granulysin+ and granulysin− T cells are indicated (Wilcoxon signed-rank test).
CD4+ T cell lines reduce Mtb burden in infected target cells in a granulysin-dependent fashion
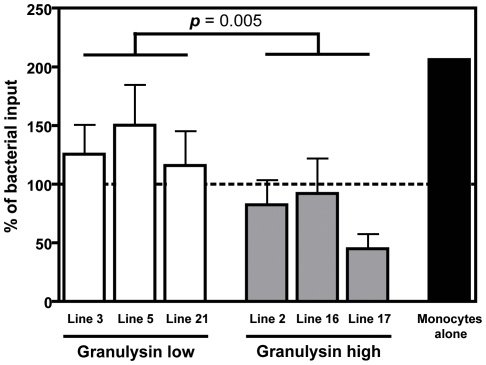
Granulysin has been demonstrated to reduce Mtb burden in infected target cells [22]. We therefore infected monocytes and cocultured them with autologous granulysin high or low T cell lines (Figure 5). Growth of Mtb was significantly decreased in cultures with granulysin-high, compared to granulysin-low, T cell lines reemphasizing their important role in the control of Mtb replication.
Figure 5. Differential effect of granulysin high- and low-expressing T cells on Mtb replication.
Monocytes (1×104) were infected with Mycobacterium tuberculosis (Mtb) 37Rv (multiplicity of infection, MOI = 5) for 24 h. Infected cells were incubated alone (black bar), or with autologous T cells expressing high and low levels of granulysin (effector∶target ratio = 10∶1). Three granulysin-low (white bars) and three granulysin-high (grey bars) T cell lines were used. After 24 h of co-culture, cells were lysed and lysates were serially diluted and plated into 7H11 agar. Colony forming units (CFU) were counted after 4 weeks. Percentages of bacterial input were calculated and are arranged in granulysin-high and -low groups. Groups were compared by Mann-Whitney U test. Dotted line represents bacterial inoculum (5×104 bacteria, 100%).
Discussion
CTL play an essential role in the immune control of TB [18]. Principally, CTL use two major pathways for elimination of infected target cells. Surface expression of FAS-L causes apoptosis in target cells expressing FAS. Exocytosis of granular content into the immunologic synapse, between CTL and target cells, causes lysis [38]. Effector proteins contained within cytotoxic granules include perforin, granzymes and granulysin [39]. Granulysin has direct antimicrobial activity against a wide spectrum of bacteria, parasites and fungi [22]. Notably, even though CTL activity is typically associated with CD8+ T cells, several reports have demonstrated Mtb-associated CTL activity in CD4+ T cells in TB [21], [40], [41]. Here we analyzed granulysin-expressing CD4+ T cells in childhood/adolescent TB and harnessed this T cell set for differential diagnosis of TB [31]. We detected profound induction of memory T cells defined as CD4+ CD45RO+ T cells after restimulation with Mtb antigens (Figure 1A,B). Restimulation significantly increased the frequencies of CD4+ CD45RO+ memory T cells in all study groups with Mtb infection, with or without TB drug therapy.
Study participants infected with NTM showed heightened frequencies of memory T cells after restimulation with the crossreactive PPD, but not after restimulation with Mtb-specific ESAT6-CFP10. In controls, frequencies of memory T cells remained unchanged after restimulation.
A possible reason for the marked induction of CD45RO+ T cells during 7-day culture could be the recruitment of naive cells to the memory compartment. We consider this possibility unlikely because CD45RO+ memory T cells were not induced by the Mtb-specific antigen ESAT-CFP10 in controls and in individuals infected with NTM. To verify whether only T cells of the memory compartment were induced by restimulation, we performed proliferation assays, which provided compelling evidence that memory T cells exclusively proliferated after restimulation with PPD and ESAT6-CFP10 (Figure 1C). Induction of CD8+ CD45RO+ memory cells could not be detected and frequencies of CD8+ T cells decreased significantly after stimulation (data not shown). Granulysin expression by T cells plays a crucial role in immune defence against TB [18], [42]. Yet, the 7-day restimulation assay is highly sophisticated and hence, in its present form, not ready for point-of-care diagnosis of childhood/adolescent TB.
Granulysin induction in memory T cells of study participants infected with Mtb, with or without TB drug therapy, was profound (p<0.0001) after PPD or ESAT6-CFP10 restimulation as compared to controls (Figure 2B). This elevated granulysin remained high in individuals newly diagnosed with active TB (p<0.001) or LTBI (p<0.01) as compared to controls (Figure 2C). Better measures for diagnosis of childhood TB are urgently needed [11]. Our data favour a 7-day in vitro restimulation assay for TB diagnosis in children. Flow cytometry has been exploited for TB diagnosis by numerous groups [43], [44], [45]. Most studies focused on intracellular IFNγ and tumour necrosis factor-alpha (TNFα) expression after restimulation, which increased sensitivity over IGRA. Despite the fact that our study was performed in a low-incidence high-income country, where the number of study participants was limited, our findings, suggest the inclusion of granulysin in flow cytometry-based assays as a diagnostic marker for childhood TB. We did not detect any correlation between antigen-specific granulysin expression of CD4+ CD45RO+ memory T cells and age distribution. Since age has been reported as one of the most important risk factors in childhood/adolescent TB, further studies comparing granulysin in different age groups would be crucial [46].
Consistent with previous studies from our group, the 7-day in vitro restimulation assay caused profound reactivation of CD45RO+ memory T cells [31]. These CD4+ CD45RO+ memory T cells were the main source of granulysin as indicated by exclusive expression in CD4+ CD45RO+ T cells undergoing cell division (Figure S1).
Additional experiments revealed an equal contribution of central and effector memory T cells after PPD re-stimulation and a preponderance of effector memory CD4+ T cells after ESAT6-CFP10 restimulation (Figure S2). This could be explained by superior processing of the recombinant protein resulting in faster activation and transition of resting central effector memory T cells into an effector memory phenotype. These findings are consistent with the data of others [47].
Di Liberto et al. [48] suggested that serum concentration of granulysin in children could be exploited for monitoring outcome of drug therapy in TB. In this study the serum concentration of granulysin before and after drug therapy differed. This effect was not seen at the T cell level in our study (Figure 2). On the contrary, our data revealed a constant high frequency of granulysin+ memory T cells even 20 months after termination of drug therapy (Figure 3A). Our data emphasize that granulysin is expressed by long-lived memory T cells. Hence its potential as immunologic marker for long-lived memory T cells in TB, should be exploited. In support of this notion, a recent report has shown that CD4+ and CD8+ T cells of BCG-vaccinated infants express granulysin after ex vivo stimulation with either BCG or PPD [49]. Moreover, vaccination with recombinant M. smegmatis, coexpressing granulysin and IL-12, reduced pulmonary CFU in lungs of Mtb-challenged mice [50].
A proportion of the memory T cell pool coexpressed IFNγ and granulysin (Figure 3B). Coexpression of these two key molecules in immune defense against Mtb has previously been shown for T cell clones [41], [51]. Our study identifies, for the first time, this T cell subset in children/adolescents after TB drug therapy and a shift from granulysin/IFNγ double-positive CD4+ T cells towards single-granulysin+ memory CD4+ T cells, over time (Figure 3B).
The phenotype of human CD4+ CTL has not been investigated in detail. Thus, we characterized additional surface markers and other cytotoxic proteins in granulysin+ CD4+ T cells (Figure 4). Amongst chemokine receptors characteristic for type 1 helper T cells, CXCR3 was significantly upregulated on granulysin+ T cells, whereas CCR5 expression remained unchanged. Bastian et al. [47], found elevated expression of CXCR3 as well as CCR5 on the surface of CD4+ CTL after stimulation with crude Mtb cell wall extracts. Increased expression of activation markers, including CD40L, CTLA-4 and HLA-DR, indicates that granulysin-producing CD4+ T cells are highly activated (Figure 4). Expression of the costimulatory surface molecule CD40L on CD4+ CTL has not been described before. Expression of CTLA-4 on Mtb-specific CD4+ T cell clones has been found to inhibit lytic activity after crosslinking [52]. Thus, CTLA-4 is likely involved in the regulation of CD4+ CTL.
Our experiments focused on granulysin expression by CD4+ CTL. Two other major cytolytic proteins in the lytic granule, perforin and granzyme B, were found to be coexpressed with granulysin (Figure 4) [22], [39]. This confirms previous observations showing that CD4+ T cells upregulate perforin and granzyme B after in vitro restimulation with Mtb [21]. This induction of granzyme B and perforin supports a cytotoxic function of these granulysin-expressing memory CD4+ T cells in TB. This notion could be further strengthened by the co-culture experiments with granulysin-expressing CD4+ T cell lines (Figure 5). As discussed above, the second pathway for target cell killing by CTL is mediated by CD95/CD95L interactions [53]. This route of induced apoptotic cell death can be excluded in our assay since CD95L on the cell surface of granulysin+ T cells was not detected (data not shown). Consistent with previous studies we assume that CD4+ CTL primarily use the cytotoxic granule mechanism rather than the CD95-dependent pathway for target cell killing [54], [55], [56].
In conclusion, we describe antigen-specific expression of granulysin by long-lived central and effector memory CD4+ T cells in Mtb-infected children and adolescents. Hence, we suggest further exploring the potential of this immune marker for diagnosis of childhood/adolescent TB.
Supporting Information
Induction of granulysin exclusively in proliferating antigen-specific CD45RO+ memory T cells. Representative dot plots (top) and histograms (bottom) of frequencies of carboxyfluorescein diacetate succinimidyl ester (CFSE)high and CFSElow populations and percentages of CD4+ CD45RO+ T cells expressing granulysin in active tuberculosis (TB) after restimulation with purified protein derivative (PPD) or ESAT6-CFP10. As positive control (far right) cells were incubated with 5 µg/ml phytohemagglutinin (PHA). Results showing are representative for six independent experiments.
(TIF)
Memory phenotype of granulysin-expressing CD4+ T cells in children/adolescents. Peripheral blood mononuclear cells (PBMC) of active tuberculosis (TB) and latent TB infection (LTBI) were restimulated with purified protein derivative (PPD) (left) or ESAT6-CFP10 (right). Cells were stained for granulysin, CD45RA and CCR7 and granulysin+ cells grouped based on distribution of surface markers. Means for each group and significant differences between central memory T cells (Tcm) and effector memory T cells (Tem) are indicated (Wilcoxon signed-rank test).
(TIF)
Acknowledgments
We thank Drs. Tom H. M. Ottenhoff and Kees L. C. M. Franken, for supplying ESAT-6 and CFP10 antigens and M. L. Grossman for help in preparing the manuscript.
Meetings
Oral presentation by Stefan H. E. Kaufmann: The content of this manuscript has been previously presented at the International Union Against Tuberculosis and Lung Disease (IUTLD), 41st Union World Conference on Lung Health, 11–15 November, 2010 in Berlin, Germany.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This study was funded by intramural funds of the Max Planck Institute for Infection Biology and received partial financial support from the European Commission's Framework Programme 7 “NEWTBVAC” (Health-F3-2009-241745; URL of Framework 7 Project NEWTBVAC: http://ec.europa.eu/research/health/infectious-diseases/poverty-diseases/projects/205_en.htm) and the Bill and Melinda Gates Foundation, Grand Challenges in Global Health (BMGF GC6-74 (no. 37772; URL of BMGFGC Projects http://www.grandchallenges.org/Pages/BrowseByGoal.aspx)). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Dolin PJ, Raviglione MC, Kochi A. Global tuberculosis incidence and mortality during 1990–2000. Bull World Health Organ. 1994;72:213–220. [PMC free article] [PubMed] [Google Scholar]
- 2.Nelson LJ, Wells CD. Global epidemiology of childhood tuberculosis. Int J Tuberc Lung Dis. 2004;8:636–647. [PubMed] [Google Scholar]
- 3.Walls T, Shingadia D. Global epidemiology of paediatric tuberculosis. J Infect. 2004;48:13–22. doi: 10.1016/s0163-4453(03)00121-x. [DOI] [PubMed] [Google Scholar]
- 4.Marais BJ, Gie RP, Schaaf HS, Hesseling AC, Obihara CC, et al. The natural history of childhood intra-thoracic tuberculosis: a critical review of literature from the pre-chemotherapy era. Int J Tuberc Lung Dis. 2004;8:392–402. [PubMed] [Google Scholar]
- 5.Beyers N, Gie RP, Schaaf HS, Van Zyl S, Talent JM, et al. A prospective evaluation of children under the age of 5 years living in the same household as adults with recently diagnosed pulmonary tuberculosis. Int J Tuberc Lung Dis. 1997;1:38–43. [PubMed] [Google Scholar]
- 6.Jacobs RF, Starke JR. Tuberculosis in children. Med Clin North Am. 1993;77:1335–1351. doi: 10.1016/s0025-7125(16)30197-3. [DOI] [PubMed] [Google Scholar]
- 7.Starke JR. Pediatric tuberculosis: time for a new approach. Tuberculosis (Edinb) 2003;83:208–212. doi: 10.1016/s1472-9792(02)00088-4. [DOI] [PubMed] [Google Scholar]
- 8.Dinnes J, Deeks J, Kunst H, Gibson A, Cummins E, et al. A systematic review of rapid diagnostic tests for the detection of tuberculosis infection. Health Technol Assess. 2007;11:1–196. doi: 10.3310/hta11030. [DOI] [PubMed] [Google Scholar]
- 9.Pai M, Riley LW, Colford JM., Jr Interferon-gamma assays in the immunodiagnosis of tuberculosis: a systematic review. Lancet Infect Dis. 2004;4:761–776. doi: 10.1016/S1473-3099(04)01206-X. [DOI] [PubMed] [Google Scholar]
- 10.Detjen AK, Loebenberg L, Grewal HM, Stanley K, Gutschmidt A, et al. Short-term reproducibility of a commercial interferon gamma release assay. Clin Vaccine Immunol. 2009;16:1170–1175. doi: 10.1128/CVI.00168-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rigouts L. Clinical practice: diagnosis of childhood tuberculosis. Eur J Pediatr. 2009;168:1285–1290. doi: 10.1007/s00431-009-0988-y. [DOI] [PubMed] [Google Scholar]
- 12.Muller I, Cobbold SP, Waldmann H, Kaufmann SH. Impaired resistance to Mycobacterium tuberculosis infection after selective in vivo depletion of L3T4+ and Lyt-2+ T cells. Infect Immun. 1987;55:2037–2041. doi: 10.1128/iai.55.9.2037-2041.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Orme IM, Collins FM. Protection against Mycobacterium tuberculosis infection by adoptive immunotherapy. Requirement for T cell-deficient recipients. J Exp Med. 1983;158:74–83. doi: 10.1084/jem.158.1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Caruso AM, Serbina N, Klein E, Triebold K, Bloom BR, et al. Mice deficient in CD4 T cells have only transiently diminished levels of IFN-gamma, yet succumb to tuberculosis. J Immunol. 1999;162:5407–5416. [PubMed] [Google Scholar]
- 15.Boom WH, Wallis RS, Chervenak KA. Human Mycobacterium tuberculosis-reactive CD4+ T-cell clones: heterogeneity in antigen recognition, cytokine production, and cytotoxicity for mononuclear phagocytes. Infect Immun. 1991;59:2737–2743. doi: 10.1128/iai.59.8.2737-2743.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hansen PW, Petersen CM, Povlsen JV, Kristensen T. Cytotoxic human HLA class II restricted purified protein derivative-reactive T-lymphocyte clones. IV. Analysis of HLA restriction pattern and mycobacterial antigen specificity. Scand J Immunol. 1987;25:295–303. doi: 10.1111/j.1365-3083.1987.tb01075.x. [DOI] [PubMed] [Google Scholar]
- 17.Kumararatne DS, Pithie AS, Drysdale P, Gaston JS, Kiessling R, et al. Specific lysis of mycobacterial antigen-bearing macrophages by class II MHC-restricted polyclonal T cell lines in healthy donors or patients with tuberculosis. Clin Exp Immunol. 1990;80:314–323. doi: 10.1111/j.1365-2249.1990.tb03287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaufmann SH. Cell-mediated immunity: dealing a direct blow to pathogens. Curr Biol. 1999;9:R97–99. doi: 10.1016/s0960-9822(99)80059-1. [DOI] [PubMed] [Google Scholar]
- 19.Ottenhoff TH, Ab BK, Van Embden JD, Thole JE, Kiessling R. The recombinant 65-kD heat shock protein of Mycobacterium bovis Bacillus Calmette-Guerin/M. tuberculosis is a target molecule for CD4+ cytotoxic T lymphocytes that lyse human monocytes. J Exp Med. 1988;168:1947–1952. doi: 10.1084/jem.168.5.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Djoba Siawaya JF, Ruhwald M, Eugen-Olsen J, Walzl G. Correlates for disease progression and prognosis during concurrent HIV/TB infection. Int J Infect Dis. 2007;11:289–299. doi: 10.1016/j.ijid.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 21.Canaday DH, Wilkinson RJ, Li Q, Harding CV, Silver RF, et al. CD4(+) and CD8(+) T cells kill intracellular Mycobacterium tuberculosis by a perforin and Fas/Fas ligand-independent mechanism. J Immunol. 2001;167:2734–2742. doi: 10.4049/jimmunol.167.5.2734. [DOI] [PubMed] [Google Scholar]
- 22.Stenger S, Hanson DA, Teitelbaum R, Dewan P, Niazi KR, et al. An antimicrobial activity of cytolytic T cells mediated by granulysin. Science. 1998;282:121–125. doi: 10.1126/science.282.5386.121. [DOI] [PubMed] [Google Scholar]
- 23.Ernst WA, Thoma-Uszynski S, Teitelbaum R, Ko C, Hanson DA, et al. Granulysin, a T cell product, kills bacteria by altering membrane permeability. J Immunol. 2000;165:7102–7108. doi: 10.4049/jimmunol.165.12.7102. [DOI] [PubMed] [Google Scholar]
- 24.Kaspar AA, Okada S, Kumar J, Poulain FR, Drouvalakis KA, et al. A distinct pathway of cell-mediated apoptosis initiated by granulysin. J Immunol. 2001;167:350–356. doi: 10.4049/jimmunol.167.1.350. [DOI] [PubMed] [Google Scholar]
- 25.Latinovic-Golic S, Walch M, Sundstrom H, Dumrese C, Groscurth P, et al. Expression, processing and transcriptional regulation of granulysin in short-term activated human lymphocytes. BMC Immunol. 2007;8:9. doi: 10.1186/1471-2172-8-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang LP, Lyu SC, Clayberger C, Krensky AM. Granulysin-mediated tumor rejection in transgenic mice. J Immunol. 2007;178:77–84. doi: 10.4049/jimmunol.178.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Donald PR, Schaaf HS. Old and new drugs for the treatment of tuberculosis in children. Paediatr Respir Rev. 2007;8:134–141. doi: 10.1016/j.prrv.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 28.WHO. Guidance for National Tuberculosis Programmes on the management of tuberculosis in children. Chapter 1: introduction and diagnosis of tuberculosis in children. Int J Tuberc Lung Dis. 2006;10:1091–1097. [PubMed] [Google Scholar]
- 29.WHO. Guidance for national tuberculosis programmes on the management of tuberculosis in children. Chapter 2: anti-tuberculosis treatment in children. Int J Tuberc Lung Dis. 2006;10:1205–1211. [PubMed] [Google Scholar]
- 30.Comstock GW. How much isoniazid is needed for prevention of tuberculosis among immunocompetent adults? Int J Tuberc Lung Dis. 1999;3:847–850. [PubMed] [Google Scholar]
- 31.Schuck SD, Mueller H, Kunitz F, Neher A, Hoffmann H, et al. Identification of T-cell antigens specific for latent mycobacterium tuberculosis infection. PLoS One. 2009;4:e5590. doi: 10.1371/journal.pone.0005590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Franken KL, Hiemstra HS, van Meijgaarden KE, Subronto Y, den Hartigh J, et al. Purification of his-tagged proteins by immobilized chelate affinity chromatography: the benefits from the use of organic solvent. Protein Expr Purif. 2000;18:95–99. doi: 10.1006/prep.1999.1162. [DOI] [PubMed] [Google Scholar]
- 33.Rivino L, Messi M, Jarrossay D, Lanzavecchia A, Sallusto F, et al. Chemokine receptor expression identifies Pre-T helper (Th)1, Pre-Th2, and nonpolarized cells among human CD4+ central memory T cells. J Exp Med. 2004;200:725–735. doi: 10.1084/jem.20040774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Qin S, Rottman JB, Myers P, Kassam N, Weinblatt M, et al. The chemokine receptors CXCR3 and CCR5 mark subsets of T cells associated with certain inflammatory reactions. J Clin Invest. 1998;101:746–754. doi: 10.1172/JCI1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meier S, Stark R, Frentsch M, Thiel A. The influence of different stimulation conditions on the assessment of antigen-induced CD154 expression on CD4+ T cells. Cytometry A. 2008;73:1035–1042. doi: 10.1002/cyto.a.20640. [DOI] [PubMed] [Google Scholar]
- 36.Noel PJ, Boise LH, Thompson CB. Regulation of T cell activation by CD28 and CTLA4. Adv Exp Med Biol. 1996;406:209–217. doi: 10.1007/978-1-4899-0274-0_22. [DOI] [PubMed] [Google Scholar]
- 37.Kamoun M, Zerva L, Sloan S, Zmijewski C, Monos D, et al. Induction of HLA class II molecules on human T cells: relationship to immunoregulation and the pathogenesis of AIDS. DNA Cell Biol. 1992;11:265–268. doi: 10.1089/dna.1992.11.265. [DOI] [PubMed] [Google Scholar]
- 38.Russell JH, Ley TJ. Lymphocyte-mediated cytotoxicity. Annu Rev Immunol. 2002;20:323–370. doi: 10.1146/annurev.immunol.20.100201.131730. [DOI] [PubMed] [Google Scholar]
- 39.Lieberman J. The ABCs of granule-mediated cytotoxicity: new weapons in the arsenal. Nat Rev Immunol. 2003;3:361–370. doi: 10.1038/nri1083. [DOI] [PubMed] [Google Scholar]
- 40.Lewinsohn DM, Bement TT, Xu J, Lynch DH, Grabstein KH, et al. Human purified protein derivative-specific CD4+ T cells use both CD95-dependent and CD95-independent cytolytic mechanisms. J Immunol. 1998;160:2374–2379. [PubMed] [Google Scholar]
- 41.Klucar P, Barnes PF, Kong Y, Samten B, Tvinnereim A, et al. Characterization of effector functions of human peptide-specific CD4+ T-cell clones for an intracellular pathogen. Hum Immunol. 2008;69:475–483. doi: 10.1016/j.humimm.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 42.Stenger S. Cytolytic T cells in the immune response to mycobacterium tuberculosis. Scand J Infect Dis. 2001;33:483–487. doi: 10.1080/00365540110026584. [DOI] [PubMed] [Google Scholar]
- 43.Nemeth J, Winkler HM, Karlhofer F, Selenko-Gebauer N, Graninger W, et al. T cells co-producing Mycobacterium tuberculosis-specific type 1 cytokines for the diagnosis of latent tuberculosis. Eur Cytokine Netw. 2010;21:34–39. doi: 10.1684/ecn.2009.0182. [DOI] [PubMed] [Google Scholar]
- 44.Cosmi L, Maggi L, Santarlasci V, Liotta F, Frosali F, et al. Detection by flow cytometry of ESAT-6- and PPD-specific circulating CD4+ T lymphocytes as a diagnostic tool for tuberculosis. Int Arch Allergy Immunol. 2007;143:1–9. doi: 10.1159/000098220. [DOI] [PubMed] [Google Scholar]
- 45.Hughes AJ, Hutchinson P, Gooding T, Freezer NJ, Holdsworth SR, et al. Diagnosis of Mycobacterium tuberculosis infection using ESAT-6 and intracellular cytokine cytometry. Clin Exp Immunol. 2005;142:132–139. doi: 10.1111/j.1365-2249.2005.02884.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wood R, Liang H, Wu H, Middelkoop K, Oni T, et al. Changing prevalence of tuberculosis infection with increasing age in high-burden townships in South Africa. Int J Tuberc Lung Dis. 2010;14:406–412. [PMC free article] [PubMed] [Google Scholar]
- 47.Bastian M, Braun T, Bruns H, Rollinghoff M, Stenger S. Mycobacterial lipopeptides elicit CD4+ CTLs in Mycobacterium tuberculosis-infected humans. J Immunol. 2008;180:3436–3446. doi: 10.4049/jimmunol.180.5.3436. [DOI] [PubMed] [Google Scholar]
- 48.Di Liberto D, Buccheri S, Caccamo N, Meraviglia S, Romano A, et al. Decreased serum granulysin levels in childhood tuberculosis which reverse after therapy. Tuberculosis (Edinb) 2007;87:322–328. doi: 10.1016/j.tube.2007.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Semple PL, Watkins M, Davids V, Krensky AM, Hanekom WA, et al. Induction of granulysin and perforin cytolytic mediator expression in 10-week-old infants vaccinated with BCG at birth. Clin Dev Immunol. 2011;2011:438463. doi: 10.1155/2011/438463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang C, He YL, Zhang L, Xu L, Yi Z, et al. GLS/IL-12-modified Mycobacterium smegmatis as a novel anti-tuberculosis immunotherapeutic vaccine. Int J Tuberc Lung Dis. 2009;13:1360–1366. [PubMed] [Google Scholar]
- 51.Mutis T, Cornelisse YE, Ottenhoff TH. Mycobacteria induce CD4+ T cells that are cytotoxic and display Th1-like cytokine secretion profile: heterogeneity in cytotoxic activity and cytokine secretion levels. Eur J Immunol. 1993;23:2189–2195. doi: 10.1002/eji.1830230921. [DOI] [PubMed] [Google Scholar]
- 52.Merlo A, Saverino D, Tenca C, Grossi CE, Bruno S, et al. CD85/LIR-1/ILT2 and CD152 (cytotoxic T lymphocyte antigen 4) inhibitory molecules down-regulate the cytolytic activity of human CD4+ T-cell clones specific for Mycobacterium tuberculosis. Infect Immun. 2001;69:6022–6029. doi: 10.1128/IAI.69.10.6022-6029.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Berke G. The CTL's kiss of death. Cell. 1995;81:9–12. doi: 10.1016/0092-8674(95)90365-8. [DOI] [PubMed] [Google Scholar]
- 54.Williams NS, Engelhard VH. Identification of a population of CD4+ CTL that utilizes a perforin- rather than a Fas ligand-dependent cytotoxic mechanism. J Immunol. 1996;156:153–159. [PubMed] [Google Scholar]
- 55.Yasukawa M, Ohminami H, Arai J, Kasahara Y, Ishida Y, et al. Granule exocytosis, and not the fas/fas ligand system, is the main pathway of cytotoxicity mediated by alloantigen-specific CD4(+) as well as CD8(+) cytotoxic T lymphocytes in humans. Blood. 2000;95:2352–2355. [PubMed] [Google Scholar]
- 56.Norris PJ, Sumaroka M, Brander C, Moffett HF, Boswell SL, et al. Multiple effector functions mediated by human immunodeficiency virus-specific CD4(+) T-cell clones. J Virol. 2001;75:9771–9779. doi: 10.1128/JVI.75.20.9771-9779.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Induction of granulysin exclusively in proliferating antigen-specific CD45RO+ memory T cells. Representative dot plots (top) and histograms (bottom) of frequencies of carboxyfluorescein diacetate succinimidyl ester (CFSE)high and CFSElow populations and percentages of CD4+ CD45RO+ T cells expressing granulysin in active tuberculosis (TB) after restimulation with purified protein derivative (PPD) or ESAT6-CFP10. As positive control (far right) cells were incubated with 5 µg/ml phytohemagglutinin (PHA). Results showing are representative for six independent experiments.
(TIF)
Memory phenotype of granulysin-expressing CD4+ T cells in children/adolescents. Peripheral blood mononuclear cells (PBMC) of active tuberculosis (TB) and latent TB infection (LTBI) were restimulated with purified protein derivative (PPD) (left) or ESAT6-CFP10 (right). Cells were stained for granulysin, CD45RA and CCR7 and granulysin+ cells grouped based on distribution of surface markers. Means for each group and significant differences between central memory T cells (Tcm) and effector memory T cells (Tem) are indicated (Wilcoxon signed-rank test).
(TIF)