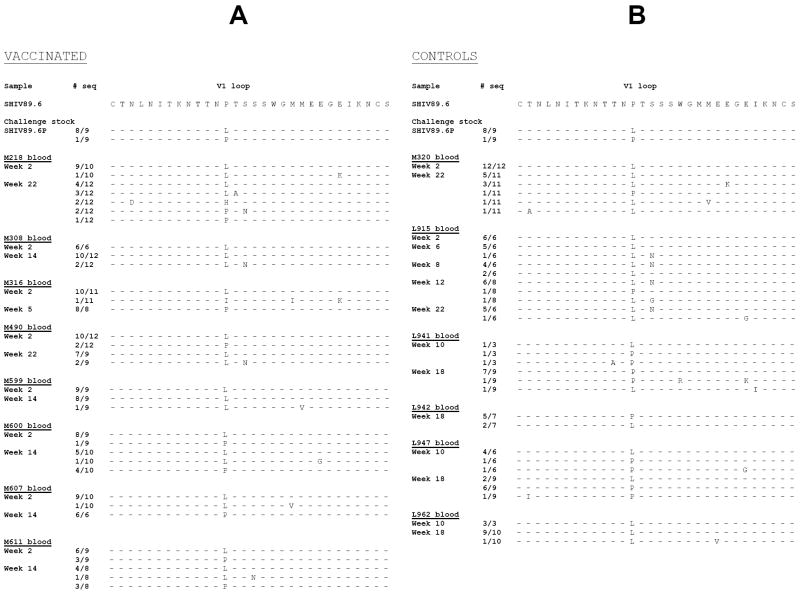
Figure 3. Viral variants within the V1 region following challenge exposure to SHIV89.6P.
Putative amino acid sequence of the SHIV89.6P V1 region obtained by DNA sequencing of plasma viral RNA following RT/PCR. The putative amino acid sequences of V1 in the vaccinated (A) and control (B) macaques were aligned with the V1 region of the SHIV89.6 used in the Tev vaccine. The number of viral clones sequenced is depicted on the left of each figure.