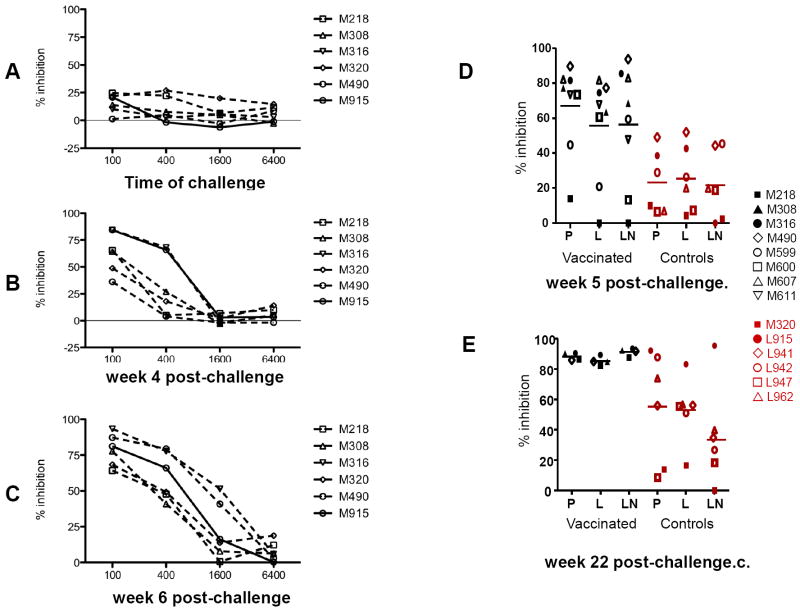
Figure 5. Sensitivity to ADCVI of SHIV 89.6 viral variants.
SHIV89.6P-infected human CD4+ lymphocyte target cells were incubated with human PBMC effector cells (E:T = 10:1) and with plasma at the dilutions indicated. Eight days later, supernatant fluid p27Gag was determined by ELISA, and virus inhibition was determined as described in the Methods. Data are representative of several experiments, each performed in duplicate. Antibody-dependent cell-mediated virus inhibition (ADCVI) activity in plasma (1:100 dilution) of the animals was performed at first using the SHIV89.6P before immunization week -2 (data not shown), or at time of challenge (A), or thereafter at week 4 (B) and 6 (C). ADCVI titers to the SHIV89.6 viral variants a week 5 (D) and 22 (E) post challenge.