Abstract
A range of early circumstances surrounding the birth of a child affects peripartum hormones, parental behavior and infant wellbeing. One of these factors, which may lead to postpartum depression, is the mode of delivery: vaginal delivery (VD) or cesarean section delivery (CSD). To test the hypothesis that CSD mothers would be less responsive to own baby-cry stimuli than VD mothers in the immediate postpartum period, we conducted functional magnetic resonance imaging, 2–4 weeks after delivery, of the brains of six mothers who delivered vaginally and six who had an elective CSD. VD mothers’ brains were significantly more responsive than CSD mothers’ brains to their own baby-cry in the superior and middle temporal gyri, superior frontal gyrus, medial fusiform gyrus, superior parietal lobe, as well as regions of the caudate, thalamus, hypothalamus, amygdala and pons. Also, within preferentially active regions of VD brains, there were correlations across all 12 mothers with out-of-magnet variables. These include correlations between own baby-cry responses in the left and right lenticular nuclei and parental preoccupations (r = .64, p < .05 and .67, p < .05 respectively), as well as in the superior frontal cortex and Beck depression inventory (r = .78, p < .01). First this suggests that VD mothers are more sensitive to own baby-cry than CSD mothers in the early postpartum in sensory processing, empathy, arousal, motivation, reward and habit-regulation circuits. Second, independent of mode of delivery, parental worries and mood are related to specific brain activations in response to own baby-cry.
Keywords: Parenting, cesarean section, maternal behavior, brain imaging, fMRI, empathy, infant
The critical capacity of adults to develop the thoughts and behaviors needed for parents to care successfully for their newborn infants is supported by specific brain circuits. Animal research suggests that networks of highly conserved hypothalamic–midbrain–limbic–paralimbic–cortical circuits act in concert via a range of hormones including oxytocin to support aspects of parent-response to infants (Leckman & Herman, 2002; Numan, 2007). In humans, functional magnetic resonance imaging (fMRI) studies have shown that infant stimuli activate brain circuitry that processes emotions, motivation, attention, and empathy (Swain & Lorberbaum, 2008; Swain, Lorberbaum, Kose, & Strathearn, 2007). Among the key factors that influence these brain circuits, the mode of delivery is important in the development of maternal behaviors.
The experience of childbirth by vaginal delivery (VD) compared with cesarean section delivery (CSD) uniquely involves the pulsatile release of oxytocin from the posterior pituitary, uterine contractions and vagino-cervical stimulation. Oxytocin is a key mediator of maternal behavior in animals (Kendrick, 2000). Indeed, across experimental models including cesarean sectioned rats (Morgan, Fleming, & Stern, 1992; Yeo & Keverne, 1986), sheep (Kendrick, Levy, & Keverne, 1992), goats (Poindron, 2005; Romeyer, Poindron, Porter, Levy, & Orgeur, 1994) and horses (Porter, Duchamp, Nowak, & Daels, 2002), vaginocervical stimulation improves the acquisition of maternal behavior. This supports the notion that cesarean delivery, which deprives mothers of this stimulation and associated neurohormonal experiences, might decrease the responsiveness of the human maternal brain in the early postpartum.
CSD is a surgical procedure, in which delivery occurs via incisions in the abdominal and uterine wall. It is considered necessary under some conditions to protect the health or survival of infant or mother (Murphy, 2001; Murphy, Liebling, Patel, Verity, & Swingler, 2003), but it is controversially linked with postpartum depression (Carter, Frampton, & Mulder, 2006; Lobel & DeLuca, 2007). The occurrence of CSD has increased precipitously in the US from 4.5% of all deliveries in 1965 to a recent high of 29.1% (Martin et al., 2006).
CSD is a perinatal event that could influence maternal brain function by altering the usual neurohormonal events of VD. To investigate this, we hypothesize that maternal brain responses to own baby-cry in the early postpartum are higher in VD mothers than CSD mothers. Specifically, we expect more activity with mothers who experienced VD and the associated vagino-cervical stimulation compared with CSD mothers in the same emotion-regulation circuits suggested by Kendrick’s model of the actions of oxytocin on sociosexual behaviors (Kendrick, 2000) that may relate to the vagino-cervical stimulation of VD. Brain areas that might differ between groups include the hormone regulation regions of the hypothalamus, motivation and reward centers of the midbrain and basal ganglia and cortical regulation regions of the cingulate. However, not wishing to limit the scope of this exploratory analysis, we performed whole brain analyses for the purpose of hypothesis generation for subsequent studies. We also hypothesize that within the regions that are more active in VD mothers, responses to own babycry correlate with measures of parental worry and mood.
Materials and methods
Participants
A group of 12 right-handed, new mothers having their first baby were recruited from the Yale New Haven Hospital’s postpartum wards to voluntarily participate in this fMRI study. Six of them had VD deliveries (aged 19.6–36.5 years, mean = 29.1; SD = 6.4) and six were CSD (aged 24.2–47.1 years, mean = 33; SD = 7.7), which was planned for convenience. None of them reported birth complications. All mothers elected to breastfeed their newborn infant. There were no significant differences between the ages of the two groups or the number of years of education (VD: 17.2 years; SD = 4.1 and CSD: 18.8 SD = 3.1) with most subjects (10/12) being college educated and with 2 high school graduates – both in the VD group. All families lived in houses or apartments in middle-to-upper class neigh-borhoods, and all parents were married and cohabiting. Each participant gave written, informed consent before participating. By self-report, no participants suffered current psychiatric diagnoses, none of them were taking psychiatric prescription medications within 2 weeks of the experiment and none had pregnancy complications. Each completed the semi-structured Yale Inventory of Parental Thoughts and Actions (YIPTA; Feldman, Weller, Leckman, Kuint, & Eidelman, 1999; Leckman et al., 2004; Leckman et al., 1999; Mayes, Swain, Feldman, & Leckman, 2008). This scale provides a validated measure of parental worries, comprising a range of anxious intrusive thoughts and harm avoidant behaviors (AITHAR). Such thoughts and behaviors include concerns about the baby’s physical environment and things being ‘just right.’ They also completed the Beck Depression Inventory (BDI), a well-validated and extensively used measure of depressed mood (Beck, Epstein, Brown, & Steer, 1988). They each received a compensation of $75 for each fMRI brain-scan and $50 for self-reports and interviews. The study was approved by the Yale University School of Medicine Human Investigations Committee.
Design of fMRI study
Maternal fMRI scans were all conducted 2–4 weeks postpartum (mean = 21.6 days postpartum, SD = 5.2, range = 16–27). During recruitment, participants were told that they would be interviewed and have brain scans to investigate the physiological and psychological brain basis of becoming a new parent. Each family was lent a digital audio recorder so that they could make digital audio recordings of their baby’s cry within the first 2 weeks. They were asked to record cries during the mild discomfort of a diaper change, but not while the baby was in pain or hungry. The digital cry recordings were uploaded and edited using computer software (Cool Edit Pro Version 2.0, Syntrillium Software, Phoenix, AZ). Building on previous parental brain imaging study design (Lorberbaum et al., 2002), baby-cry stimuli consisted of blocks of 30-second cries, prepared by removing all non-baby-cry sounds such as background conversations, baby-gurgles and grunts. Control sounds were made by replicating cry bursts with white noise sound bursts that simulated each cry’s temporal pattern and matched the total root-mean-square sound intensity and range of frequencies. Thus, the resulting electrical static-like control sounds controlled for stimulus volume, pattern and frequency range, without sounding anything like an actual baby-cry. In addition, beeps of .4 second duration were inserted at 10, 20 and 30 seconds of each cry and control block in order to act as prompts for button-press responses to ensure attention during the brain imaging (described below). Because each own baby-cry varied according to the individual characteristics of each baby, the own baby-cry and control-sound blocks were necessarily unique to each mother. However, the ‘wah’ frequency was kept to between 1 and 2 Hz (.5–1 ‘wah’s’/sec), with a ‘wah’ defined as a discrete sharp rise and immediately following fall in sound frequency. In order to address the concern that there may be a qualitative difference between cries from babies born by CSD vs. VD, we asked 7 adults to rate all of the baby-cry stimuli on a 1–10 scale of emotional intensity. An independent samples t-test revealed no significant difference between VD and CSD baby cries. The other baby-cry stimulus block was identical for each participant that we scanned. This was chosen as the cry of median intensity from 8 sample cries recorded during pilot experiments, which were rated on a scale of 1–10 by a group of 8 adults.
fMRI data acquisition
fMRI data were acquired on a Siemens Trio 3T full-body scanner (Erlangen, Germany) with echo-planar imaging of the head. Head movements were restrained with foam padding and surgical tape placed across each participant’s forehead. First, high-resolution T1-weighted anatomical images were obtained (3D MPRAGE; TR = 2530; TE = 3.66; matrix size 256 × 256; 176 slices). Then, anatomical T1-weighted echo-planar images (spin-echo; TR = 300ms; TE = 4ms; matrix size 64 × 64; 30 axial slices; 3.125-mm in-plane resolution 5-mm thick, no skip) were acquired to be coplanar with the functional scans for spatial registration. Subsequently, two BOLD-fMRI functional runs, each lasting 6 minutes and 50 seconds, were completed (echo planar T2*- weighted gradient-echo, TR = 2000 ms, TE = 30ms, flip angle = 80°, matrix size 64 × 64, 30 axial slices, 3.125-mm in-plane resolution; 5-mm thick, skip 0) to span the entire brain.
fMRI procedures
During each of the two BOLD-fMRI functional runs, participants wore padded headphones to hear 10 blocks of stimuli. Each stimulus block was 30 seconds long and composed of (1) own baby-cry, (2) other baby-cry, or (3) own baby-cry-control-sound. These blocks were arranged so participants heard each sound block a total of five times in a pseudorandom order. Each block was separated by a 10-sec rest period during which only background scanner noise could be heard. In order to balance concerns of obscuring responses in parenting circuits with either the surprise of new stimuli or losing brain responses with habituation, we exposed each subject to a 10-second clip each of the stimuli before the start of scanning. During the scan, subjects were asked to push a button with beep prompt at 10s, 20s and 30s to indicate the intensity of emotional response by pressing the button box (0 = none, 1 = a little, 2 = a lot, 3 = maximal) to ensure that they were awake and attending to the stimuli.
fMRI data analysis
Image processing, analyses, and statistical tests were performed using Brainvoyager QX (version 1.6 to 1.8) commercial software (http://www.BrainVoyager.com).
Preprocessing
Motion-corrected images were spatially smoothed using a Gaussian filter with a full-width half-maximum value of 6.25 mm. Data for each individual were aligned with high-resolution 2D and 3D anatomical images for display and localization. For group analysis, individual data sets underwent piecewise linear transformation into a proportional 3D grid defined by Talairach and Tournoux (1988) and co-registered with the high-resolution 3D data set that was re-sampled to give 1 mm3 voxels.
Statistical whole brain and region-of-interest (ROI) analysis
In the data sets, averaged group functional activation was examined using a random effects general linear model (GLM) analysis allowing pair-wise contrasts of different experimental conditions. In order to test statistical significance with correction for multiple comparisons, whole-brain voxel-based spatial cluster filter thresholding was performed (Forman et al., 1995; Goebel, Esposito, & Formisano, 2006). First an uncorrected threshold was set at p < .05. Then, thresholded maps were submitted to a whole-brain correction criterion based on the estimate of the map’s spatial smoothness and on an iterative procedure (Monte Carlo simulation) for estimating cluster-level false positive rates. After 1,000 iterations, the minimum cluster size threshold that yielded a cluster-level false-positive rate of 5% was applied to the statistical maps. This method corrects for multiple cluster tests across space (Goebel, Esposito, & Formisano, 2006). We thus rejected uncorrected activated clusters that were smaller than 813 mm3 for own baby-cry vs. control sound and 777 mm3 for own vs. other baby-cry yielding surviving corrected clusters at p < .05. Additionally, to test the significance of activity in small brain areas, we applied a small volume correction (SVC) method for false discovery rate (FDR) error at p < .05 corrected (Genovese, Lazar, & Nichols, 2002). This was implemented on Brainvoyager software and gives statistical corrections for multiple comparisons confined to a region of interest (Worsley et al., 1996). The small volume consisted of a cube (6 mm3) centered at the most significant voxel of the clusters activated at p < .05 (t > 2.22) uncorrected in the whole brain analysis. For correlations of brain activations in response to baby-cry and out-of-magnet behavioral measures, we performed random effects analysis of covariance correlations within significantly activated clusters with individual scores on the Beck Depression Inventory and the YIPTA measure of parental worries.
In order to explore maternal brain responses to baby-cry, we obtained group differences by performing two-factor analysis of variance between VD and CSD parents. There were no significant differences between VD and CSD groups in response to the control sound or the other baby-cry vs. baseline, such that the significant between-group effects for own vs. control sound and own vs. other baby-cry were due to the responses to own baby-cries. The fMRI contrasts we examined across modes of delivery were:
Baby-Cry Response (the own baby-cry vs. own baby-control-sound contrast). This contrast controls for the timing pattern and volume to reveal brain activations important to baby-cry stimuli.
Own Baby-Cry Response (the direct own baby-cry vs. other baby-cry contrast). This contrast controls for the qualities of baby-cry to reveal brain activations more purely related to the identity or ‘oneness’ of the baby-cry stimulus.
In order to test for valid correlations between own baby-cry response and behavioral measures, we only looked for correlations within ‘masked’ brain areas that were more active in response to own baby-cry in VD vs. CSD mothers. Within this mask, we performed random effects analysis of covariance between brain response to own baby-cry and AITHAR and BDI across the entire sample.
Results
Behavioral results
There were no significant differences in emotional ratings of baby-cry or control-sound stimuli between VD and CSD subjects. In addition, across all subjects, there were no significant differences between emotional response scores (0–3) between own baby-cry (2.7 ± .3) and other baby-cry (2.6 ± .5). However, there were significant differences (p < .05) between emotional response scores to baby-cry (2.7 ± .3) vs. control-sound (1.8 ± .6). With our subjects, there was not a significant difference for AITHAR (VD = 1.01 ± .29; CSD = 1.02 ± .40) and just a trend (p = .059), but not a significant group difference for VD mothers to a higher BDI than CSD mothers (VD = 7.3 ± 4.5; CSD = 4.7 ± 2.3) using independent sample t-tests.
Neuroimaging
Baby-cry responses at 2–4 weeks postpartum
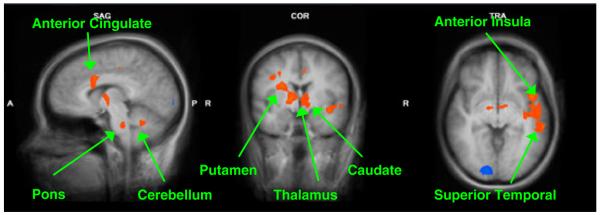
In our sample of 12 parents at 2–4 weeks postpartum, we contrasted own baby-cry vs. control-sound between VD (n = 6) and CSD (n = 6) mothers. This revealed a range of regions that were significantly more active in VD vs. CSD mothers in response to baby-cry, including cortical and subcortical structures. These included circuits of the anterior cingulate, hypothalamus, and regions of the basal ganglia. In addition, we found activations in sensory processing areas of the superior and inferior temporal cortex, frontal premotor and motor circuits and emotion regulation insular cortex, as well as thalamus, pons and regions within the cerebellum. There were no clusters that were significantly more active in CSD vs. VD mothers in our sample except in the occipital lobe (Figure 1, Table 1).
Figure 1.
Maternal brain activity of VD vs. CSD (Baby-Cry vs. Control-Sound) (x = 7, y = −3, z = −4). These are sample views of the group contrast between vaginal delivery (n = 6) and Cesarean section delivery (n = 6) for the contrast own baby-cry > control-sound (p < .005, uncorrected). Please see Table 1 for a detailed list of corrected activations
Table 1.
Baby-cry vs. control sound at 2–4 weeks postpartum (n = 12). Vaginal (n = 6) > cesarean section (n = 6)
| Parent brain region | R/L | Talairach coordinates | Peak t-score | Volume (mm3) |
|---|---|---|---|---|
| Superior Temporal Gyrus BA21 | L | −49, −11, −5 | 3.05 | 1986 |
| Medial Inferior Temporal/Fusiform BA36 | R | 21, −31, −17 | 4.17 | 832 |
| Supplementary Motor Cortex BA6 | R | 39, −4, 35 | 3.09 | 873 |
| Primary Motor Cortex BA4 | R | 24, −17, 50 | 3.06 | 788 |
| L | −18, −19, 49 | 3.57 | 841 | |
| Parietal Supramarginal Gyrus BA40 | L | −24, −43, 55 | 4.97 | 842 |
| Inferior Parietal Lobule BA7 | L | −36, −52, 57 | 3.09 | 840 |
| Anterior Cingulate Gyrus, BA24 | R | 12, 11, 31 | 4.26 | 1466 |
| L | −7, −7, 41 | 3.34 | 816 | |
| Dorsal Posterior Cingulate BA31 | R | 3, −22, 47 | 2.88 a | |
| L | −1, −22, 46 | 3.03 a | ||
| Insula, BA13 | L | −45, 8, −2 | 3.94 | 914 |
| Claustrum BA13 | R | 30, 4, 15 | 5.17 a | |
| Parahippcampal Gyrus BA 28 | R | 20, −19, −17 | 4.02 | 819 |
| Caudate Body | R | 14, 6, 18 | 3.83 a | |
| L | −15, 8, 13 | 4.57 a | ||
| Lenticular nucleus | R | 16, 2, 4 | 3.68 a | |
| L | −18, 5, 4 | 3.10 a | ||
| Thalamus VAN | R | 6, −7, 1 | 6.15 a | |
| L | −9, −6, −2 | 3.89 a | ||
| Hypothalamus | R | 7, −6, −3 | 2.41 a | |
| L | −9, −4, −4 | 3.20 a | ||
| Pons | R | 15, −28, −29 | 5.73 a | |
| Cerebellum, Anterior Lobe, Tonsil | R | 15, −43, −29 | 3.57 | 865 |
| L | −15, −44, −32 | 3.00 a | ||
| Cerebellar Anterior Lobe, Dentate | R | 9, −50, −26 | 3.36 a | |
| L | −6, −49, −26 | 3.41 | 867 | |
| Cerebellar Anterior Lobe, Culmen | L | −29, −43, −26 | 3.01 a |
p < 0.05, corrected by whole brain cluster filter threshold.
significantly different at p < 0.05 (FDR) – small volume corrected from region that was significantly active in the whole brain uncorrected map at p < 0.005.
Own baby-cry vs. other baby-cry response at 2–4 weeks postpartum
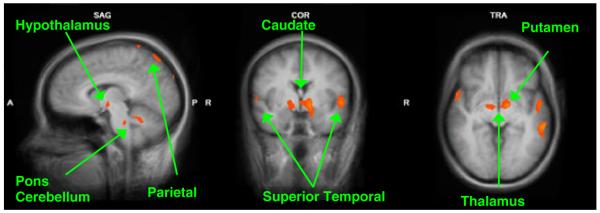
In our sample of 12 parents at 2–4 weeks postpartum, we contrasted own baby-cry vs. other baby-cry between VD (n = 6) and CSD (n = 6) mothers. This indicates maternal responses to own baby that were higher with VD vs. CSD mothers in regions including frontal cortex circuits, hypothalamus and basal ganglia. In addition, we report differences in sensory processing areas of the temporal cortex and parietal cortex as well as other subcortical regions of the caudate head, thalamus and pons. One cluster in the insula was more significantly active in CS than VD mothers. (Figure 2, Table 2).
Figure 2.
Maternal brain activity for VD vs. CSD for (Own baby-cry > Other baby-cry) (x = 7, y = −3, z = −4). These are sample views of the group comparison of mothers who delivered by vaginal delivery (n = 6) vs. Cesarean section (n = 6) for the contrast of own baby-cry vs. other baby-cry (p < .005, uncorrected). Please see Table 2 for a detailed list of corrected activations
Table 2.
Own baby-cry vs. other baby-cry at 2–4 weeks postpartum (n = 12). Vaginal (n = 6) > cesarean section (n = 6)
| Parent brain region | R/L | Talairach coordinates | Peak t-score | Volume (mm3) |
|---|---|---|---|---|
| Superior Temporal Gyrus BA22 | R | 54,10,1 | 4.29 | 803 |
| L | −51, −1,2 | 3.77 | 980 | |
| Middle Temporal Lobe BA36 | L | −60, −31, −5 | 4.64 | 836 |
| Superior Frontal Gyrus BA10 | L | −15, 56, 22 | 5.06 | 821 |
| Lateral Fusiform BA37 | R | 39, −46, −22 | 4.48 | 997 |
| L | −36, −46, −17 | 5.10 | 795 | |
| Superior Parietal Lobule BA7 | R | 15, −61, 64 | 7.30 | 1351 |
| L | −21, −63, 62 | 5.48 | 1631 | |
| Caudate Head | L | −6, 5, 1 | 2.76 a | |
| Thalamus | L | −9, −6, 2 | 3.31 | 828 |
| Hypothalamus | L | −9, −4, −6 | 4.15 a | |
| Amygdala | L | −21, 0, −16 | 3.50 a | |
| Lenticular Nucleus | R | 14, −2, 1 | 3.09 a | |
| L | −14, −1, −2 | 5.38 a | ||
| Pons | R | 2, −28, −31 | 3.30 a | |
| L | −2, −28, −29 | 3.75 a | ||
| Cesarean section (n = 6) > vaginal (n = 6) | ||||
| Insula | L | −33,24,10 | 3.89 | 792 |
p < 0.05, corrected by whole brain cluster filter threshold.
significantly different at p < 0.05 (FDR) – small volume corrected from region that was significantly active in the whole brain uncorrected map at p < 0.005.
Random effects analysis of covariance with neuroimaging and behavioral measures
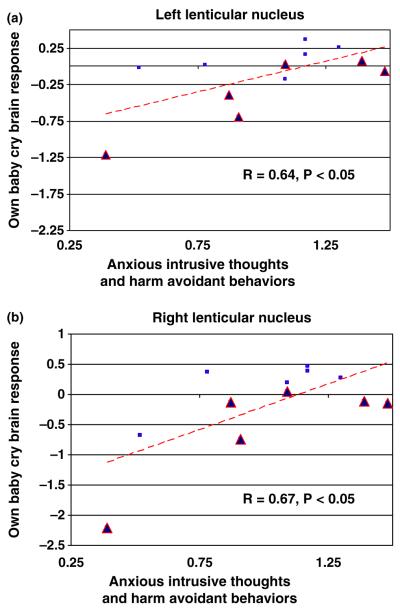
Within the regions more responsive to own baby-cry in VD vs. CSD mothers, we found responses to own baby-cry correlated with out-of-magnet measures of thoughts and mood across the entire sample of mothers. First, the measure of parental preoccupation (AITHAR) correlated with brain activity in response to own baby-cry in bilateral lenticular nuclei (r = .64, p < .05 and .67, p < .05 respectively). Second, the depression measure BDI (r = .78, p < .01) correlated with own baby-cry brain activity in a region of the superior frontal gyrus (BA10).
Discussion
The results of this study show that attending to own baby-cry evokes a unique pattern of neural responses in VD mothers as compared to CSD mothers in the early postpartum. These differences between VD and CSD mothers are obtained by comparing neural activation while parents listened to their own baby-cry and control stimuli. Brain differences between VD and CSD mothers in response to their own baby-cry may reflect the effects of vaginal delivery, associated vagino-cervical stimulation and related biobehavioral events that may contribute to mental health risks and resiliency in the mother–infant dyad. Independent of the mode of delivery, there were specific correlations across all parents between response to own baby-cry and levels of parental worry and mood suggesting the importance of these psychological dimensions to the emergence of parenting behaviors.
First, using a control stimulus consisting of pattern and volume matched white noise reveals specific responses of VD mothers to characteristics of baby-cry. These include areas associated with emotional response, such as the anterior cingulate and insula as well as areas associated with decision-making such as the posterior cingulate. Second, using a control stimulus consisting of a volume matched other baby-cry reveals increased brain response of VD mothers to the identity of the own baby. This includes areas associated with arousal and reward such as the amygdala and the head of the caudate, areas associated with the representation of the mental states of others (theory of mind) such as the superior temporal cortex, and areas associated with the regulation of repetitive thoughts and behaviors including the lenticular nucleus. Increased responses for VD vs. CSD mothers with both contrasts occurred in areas associated with neurohormonal regulation such as the hypothalamus as well as broadly important regions of the pons, thalamus and cortex.
Activations in the anterior cingulate, insula and claustrum may be important for processing of vocal emotions (Johnstone, van Reekum, Oakes, & Davidson, 2006) and integrating representations of the baby with limbic regulation of emotions (Carr, Iacoboni, Dubeau, Mazziotta, & Lenzi, 2003; Crick & Koch, 2005; Goldapple et al., 2004; Gotlib et al., 2005; Wiens, 2005). Increased activity in the anterior cingulate with VD mothers also fits with its role in selecting a response between competing thoughts and correcting errors (Posner & Rothbart, 1998). Increased activity in the insula may also reflect positive self-conscious emotions (Takahashi et al., 2008), which may be increased with VD vs. CSD mothers because of their increased sense of self-confidence (Clement, 2001). Findings in the superior temporal and frontal cortices may also reflect auditory processing and social cognition (Decety & Grezes, 2006; Saxe, 2006). Thus, in VD mothers more than CSD mothers, brain responses may be important for the emotion regulation and empathy in mothers when they hear a baby-cry that may assist them in recognizing and understanding the infant’s mental state, leading to more accurate predictions of likely infant behaviors and needs. This would assist VD mothers in carrying out appropriate caring behaviors and perform critical interpersonal tasks in parent-infant bonding (Feldman, 2007).
Sub-cortical regions such as the thalamus, caudate, and pons may mediate complex sensory integration, motivation and reward that are part of normal responses to baby-cry. Thalamic activity, in concert with cortical activity may be associated with parenting across a range of animals in which infant cry has evolved to allow infants to initiate breast-feeding and other parenting behaviors (Newman, 2007; Soltis, 2004). Perhaps thalamus activity, in unison with empathy circuits, allows parents to appreciate the discomfort of their baby indicated by a cry. The caudate may be associated with the integration of sensory input, reward expectation and detection (Delgado, 2007; Knutson & Cooper, 2005; Martin-Soelch et al., 2001) to motivate and prepare for rewarding experiences including receiving money (Peterson, 2005; Schultz, 2000), using addictive substances such as cocaine (Breiter & Rosen, 1999), sexual arousal (Karama et al., 2002) and orgasm (Komisaruk & Whipple, 2005). Future studies with parents may clarify if baby-stimuli may be similarly rewarding to parents.
VD vs. CSD activations in the hypothalamus may reflect its role in stress regulation and parenting behaviors such as breastfeeding (Numan, 2006) as well as other affiliative behaviors and attachment (Bartz & Hollander, 2006; Feldman, Weller, Zagoory-Sharon, & Levine, 2007). This occurs at least in part via the peptide hormone oxytocin (Insel & Young, 2001; Koob & Le Moal, 2001; Swain, Mayes, & Leckman, 2005) which may be altered in CSD in which the mother is deprived of the vagino-cervical stimulation and associated oxytocin (Kendrick, 2000).
Increased activity for VD mothers in parahippocampal gyrus may fit with its importance for encoding emotional memories (Phelps, 2004), which may be impaired by the post CSD pain (LaBar & Cabeza, 2006). Another mental phenomenon which might be important for early postpartum parenting is the presence of postpartum parental preoccupations (Leckman et al., 1999). Such thoughts may be supported by the same brain circuits that are involved in obsessions and compulsions such as regions of the basal ganglia (Mayes, Swain, & Leckman, 2005). In keeping with this notion, we found activations in the lenticular nucleus that are more sensitive to own baby-cry in VD mothers. It is also interesting that inferior temporal regions in the area referred to as the fusiform face area are more active in VD mothers, suggesting that own baby-cry is actually activating regions that normally process expert face information (Kanwisher & Yovel, 2006; Schultz, 2005) – perhaps in this case the baby’s face – when they listen to their own baby-cry. Finally, relative activity in VD mothers in the cerebellum may signal the importance of this structure in cognition and emotion (Ioannides & Fenwick, 2005). The only area that was more responsive to own baby-cry in CSD vs. VD mothers was in a region of the insula. This may fit with the role of anterior insula in pain processing which might be quite different after the experience of childbirth by VD vs. CSD.
Considering that the areas of response to own baby-cry in VD vs. CSD mothers may be part of critical parenting brain circuits, we tested for correlations with behavioral data within those areas. Across the entire sample, we found regions in which the sensitivity to own baby-cry correlated with AITHAR (parental-worry) bilaterally in the lenticular nuclei (Figure 3). This is consistent with the function of these regions in a range of repetitive anxious thoughts and behaviors (Mataix-Cols et al., 2004). There is also a direct correlation between BDI and brain response to own infant-cry in the superior medial frontal gyrus Brodmann area 10 (Figure 4). This area has been associated with circuits important for depression (Killgore & Yurgelun-Todd, 2006), recovery from depression (Mayberg et al., 2005), and cocaine administration (Kufahl et al., 2005). Despite these convergences of findings in this region, in our normal sample depression scores may not be truly comparable to the depression of psychiatric magnitude. Perhaps the association of activity in this area with the subclinical levels of depression we report actually reflects normal and even healthy aspects of adjusting to the new role as mother with associated stress, sleep deprivation and fatigue. Indeed, VD mothers show a trend to higher BDI scores than CSD mothers.
Figure 3.
OWN vs. OTHER baby-cry vs. parental worries in all mothers. This scatter-plot shows significant correlations between the functional magnetic resonance imaging (fMRI) response at the local maxima to own vs. other baby-cry in the left (−14, −2, 1) and right lenticular nuclei (14, −1, −2). Red triangles indicate CSD mothers, blue squares indicate VD mothers. This was the only region from table 2 (VD vs. CSD) that correlated with BDI
Figure 4.
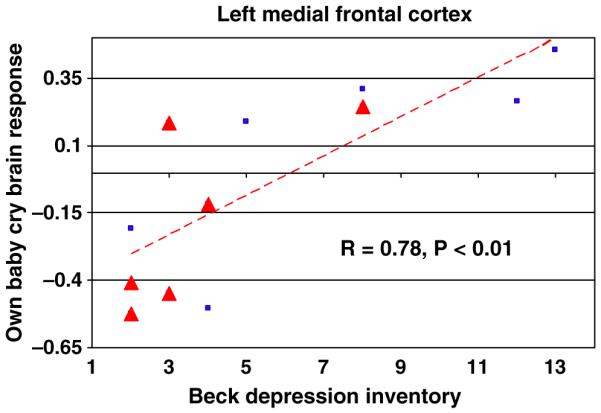
OWN > OTHER baby-cry vs. BDI in mothers (VD + CSD). This scatter-plot shows the relationship between BDI scores and the functional magnetic resonance imaging (fMRI) response at the local maxima in the left medial frontal (Brodmann Area 10, −16,56,20) region for own baby-cry vs. other baby-cry (r = .73, p < .01). Red triangles indicate CSD mothers, blue squares indicate VD mothers
Despite the statistically significant findings that we report, there are several important limitations that should be addressed in future replications. Certainly, increased sample size and repetition at different postpartum time points in the same subjects would be significant improvements. In addition, more measurements of parenting and child outcomes are needed to establish the importance of specific brain regions. Correlations with such measures could separate brain responses from simple attention and sensory processing from those related to truly relevant dimensions of parenting competence and infant outcome that may be future targets for treatment in depressed or at-risk parents.
As we hypothesize in this fMRI study – the first to compare brain responses to own baby-cry stimuli in mothers according to delivery – certain brain regions respond to own baby-cry stimuli more in VD vs. CSD mothers. Furthermore, the responses of some of those parental response areas vary according to parental worry and mood. It is impressive that at 2–4 weeks postpartum, the effects of mode of delivery on brain responsiveness to own baby-cry are significant. There are many reasonable explanations for the complex biopsychosocial differences associated with VD vs. CSD, but it is tempting to speculate about the differential release of oxytocin with the vagino-cevical stimulation of VD that has been studied in animal models (Kendrick, 2000). It may be possible to approach this question in humans by comparing brain function of breastfeeding and formula feeding mothers. Recently, oxytocin has been linked in humans with a set of maternal behaviors in the early postpartum (Feldman, Weller, Zagoory-Sharon, & Levine, 2007) This demonstration of differences in brain responses to infants across groups that likely differ in oxytocin is consistent, but further work is needed to clarify the link between mode of delivery, oxytocin, and postpartum anxiety and mood problems that may increase the risks for postpartum problems. This may lead to treatment studies with such interventions as increased maternal–infant contact with CSD mothers. This has been shown to improve infant physiology, development, maternal mood, and mother–infant relationships in general (Feldman, 2004).
It is of particular importance to study the CSD at this time because of its rising rates in Mexico, Canada, and some European, Asian, and South American countries such as Brazil, where the rate in public hospitals is as high as 80% (Kambo, Bedi, Dhillon, & Saxena, 2002; Lin & Xirasagar, 2004; Matthews et al., 2003; Trujillo-Hernandez et al., 2002). Increases in CSD rates may be attributed to a range of factors, including obstetric practice, litigation issues, financial incentives, and shifting trends among women giving birth such as advancing age and excessive pregnancy weight gain (Luthy, Malmgren, Zingheim, & Leininger, 2003; Rosenberg, Garbers, Lipkind, & Chiasson, 2005; Villar et al., 2006; Xirasagar, Lin, & Liu, 2006), as well as issues of choice (Declercq, Menacker, & Macdorman, 2006). If our data is replicated, CSD mothers are less sensitive to their infant and this may contribute to the possible link between CSD and a range of adverse psychosocial effects (Clement, 2001), including postpartum depression (PPD) (Lobel & DeLuca, 2007). Maternal PPD predicts poorer infant cognitive outcome at 18 months (Murray & Cooper, 2003) and later time-points such as 7 years postpartum (Kim-Cohen, Moffitt, Taylor, Pawlby, & Caspi, 2005). Furthermore, follow-up data on the offspring of depressed and anxious mothers indicate increased mental health risks across generations (Brown, Bifulco, & Harris, 1987; Heim, Owens, Plotsky, & Nemeroff, 1997; Kendler, Kessler, Neale, Heath, & Eaves, 1993; Sroufe, Carlson, Levy, & Egeland, 1999). Thus, building on the animal and human literature, further study is needed of the effects of CSD and other stressors on parenting and infant wellbeing.
Acknowledgements
The authors wish to acknowledge Virginia Eicher, Elizabeth Hoyt, Hannah Kang, Pilyoung Kim, and Nancy Thompson for research assistance and key discussions. In addition, we would like to thank Jeff Lorberbaum for his support and advice from the beginning of this project. Also, we would like to express our appreciation for financial support from the Institute for Research on Unlimited Love (unlimitedloveinstitute.org) (JFL, JES), Young Investigator Awards (JES) from the National Alliance of Research on Schizophrenia and Depression (narsad.og), NIH grants K05MH076273 (JFL) and K05DA020091 (LCM). Finally, we appreciate the assistance of the Yale Program for Risk, Resilience and Recovery and Associates of the Yale Child Study Center.
Abbreviations
- fMRI
functional magnetic resonance imaging
- VD
vaginal delivery
- CSD
cesarean section delivery
- PPD
postpartum depression
Footnotes
Conflict of interest statement: No conflicts declared.
References
- Bartz JA, Hollander E. The neuroscience of affiliation: Forging links between basic and clinical research on neuropeptides and social behavior. Hormones and Behavior. 2006;50:518–528. doi: 10.1016/j.yhbeh.2006.06.018. [DOI] [PubMed] [Google Scholar]
- Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: Psychometric properties. Journal of Consulting and Clinical Psychology. 1988;56:893–897. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- Breiter HC, Rosen BR. Functional magnetic resonance imaging of brain reward circuitry in the human. Annals of the New York Academy of Sciences. 1999;877:523–547. doi: 10.1111/j.1749-6632.1999.tb09287.x. [DOI] [PubMed] [Google Scholar]
- Brown GW, Bifulco A, Harris TO. Life events, vulnerability and onset of depression: Some refinements. British Journal of Psychiatry. 1987;150:30–42. doi: 10.1192/bjp.150.1.30. [DOI] [PubMed] [Google Scholar]
- Carr L, Iacoboni M, Dubeau MC, Mazziotta JC, Lenzi GL. Neural mechanisms of empathy in humans: A relay from neural systems for imitation to limbic areas. Proceedings of the National Academy of Sciences U S A. 2003;100:5497–5502. doi: 10.1073/pnas.0935845100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter FA, Frampton CM, Mulder RT. Cesarean section and postpartum depression: A review of the evidence examining the link. Psychosomatic Medicine. 2006;68:321–330. doi: 10.1097/01.psy.0000204787.83768.0c. [DOI] [PubMed] [Google Scholar]
- Clement S. Psychological aspects of caesarean section. Best Practice and Research. Clinical Obstetrics and Gynaecology. 2001;15:109–126. doi: 10.1053/beog.2000.0152. [DOI] [PubMed] [Google Scholar]
- Crick FC, Koch C. What is the function of the claustrum? Philosophical Transaction of the Royal Society London B: Biological Sciences. 2005;360:1271–1279. doi: 10.1098/rstb.2005.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decety J, Grezes J. The power of simulation: Imagining one’s own and other’s behavior. Brain Research. 2006;1079:4–14. doi: 10.1016/j.brainres.2005.12.115. [DOI] [PubMed] [Google Scholar]
- Declercq E, Menacker F, Macdorman M. Maternal risk profiles and the primary cesarean rate in the United States, 1991–2002. American Journal of Public Health. 2006;96:867–872. doi: 10.2105/AJPH.2004.052381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado MR. Reward-related responses in the human striatum. Annals of the New York Academy of Sciences. 2007;1104:70–88. doi: 10.1196/annals.1390.002. [DOI] [PubMed] [Google Scholar]
- Feldman R. Advances in psychology research. Vol. 27. Nova Science; Hauppage, NY: 2004. Mother–infant skin-to-skin contact and the development of emotion regulation; pp. 113–131. [Google Scholar]
- Feldman R. Parent–infant synchrony and the construction of shared timing: Physiological precursors, developmental outcomes, and risk conditions. Journal of Child Psychology and Psychiatry. 2007;48:329–54. doi: 10.1111/j.1469-7610.2006.01701.x. [DOI] [PubMed] [Google Scholar]
- Feldman R, Weller A, Leckman JF, Kuint J, Eidelman AI. The nature of the mother’s tie to her infant: Maternal bonding under conditions of proximity, separation, and potential loss. Journal of Child Psychology and Psychiatry. 1999;40:929–939. [PubMed] [Google Scholar]
- Feldman R, Weller A, Zagoory-Sharon O, Levine A. Evidence for a neuroendocrinological foundation of human affiliation: Plasma oxytocin levels across pregnancy and the postpartum period predict mother–infant bonding. Psychological Science. 2007;18:965–70. doi: 10.1111/j.1467-9280.2007.02010.x. [DOI] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): Use of a cluster-size threshold. Magnetic Resonance in Medicine. 1995;33:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. NeuroImage. 2002;15:870–878. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- Goebel R, Esposito F, Formisano E. Analysis of functional image analysis contest (FIAC) data with brainvoyager QX: From single-subject to cortically aligned group general linear model analysis and self-organizing group independent component analysis. Human Brain Mapping. 2006;27:392–401. doi: 10.1002/hbm.20249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldapple K, Segal Z, Garson C, Lau M, Bieling P, Kennedy S, Mayberg H. Modulation of cortical-limbic pathways in major depression: Treatment-specific effects of cognitive behavior therapy. Archives of General Psychiatry. 2004;61:34–41. doi: 10.1001/archpsyc.61.1.34. [DOI] [PubMed] [Google Scholar]
- Gotlib IH, Sivers H, Gabrieli JD, Whitfield-Gabrieli S, Goldin P, Minor KL, Canli T. Subgenual anterior cingulate activation to valenced emotional stimuli in major depression. Neuroreport. 2005;16:1731–1734. doi: 10.1097/01.wnr.0000183901.70030.82. [DOI] [PubMed] [Google Scholar]
- Heim C, Owens MJ, Plotsky PM, Nemeroff CB. The role of early adverse life events in the etiology of depression and posttraumatic stress disorder. Focus on corticotropin-releasing factor. Annals of the New York Academy of Sciences. 1997;821:194–207. doi: 10.1111/j.1749-6632.1997.tb48279.x. [DOI] [PubMed] [Google Scholar]
- Insel TR, Young LJ. The neurobiology of attachment. Nature Reviews. Neuroscience. 2001;2:129–136. doi: 10.1038/35053579. [DOI] [PubMed] [Google Scholar]
- Ioannides AA, Fenwick PB. Imaging cerebellum activity in real time with magnetoenceph-alographic data. Progress in Brain Research. 2005;148:139–150. doi: 10.1016/S0079-6123(04)48012-1. [DOI] [PubMed] [Google Scholar]
- Johnstone T, van Reekum CM, Oakes TR, Davidson RJ. The voice of emotion: An FMRI study of neural responses to angry and happy vocal expressions. Social Cognitive and Affective Neuroscience. 2006;1:242–249. doi: 10.1093/scan/nsl027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kambo I, Bedi N, Dhillon BS, Saxena NC. A critical appraisal of cesarean section rates at teaching hospitals in India. International Journal of Gynaecology and Obstetrics. 2002;79:151–158. doi: 10.1016/s0020-7292(02)00226-6. [DOI] [PubMed] [Google Scholar]
- Kanwisher N, Yovel G. The fusiform face area: A cortical region specialized for the perception of faces. Philosophical Transaction of the Royal Society London B: Biological Sciences. 2006;361:2109–2128. doi: 10.1098/rstb.2006.1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karama S, Lecours AR, Leroux JM, Bourgouin P, Beaudoin G, Joubert S, Beauregard M. Areas of brain activation in males and females during viewing of erotic film excerpts. Human Brain Mapping. 2002;16:1–13. doi: 10.1002/hbm.10014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Kessler RC, Neale MC, Heath AC, Eaves LJ. The prediction of major depression in women: Toward an integrated etiologic model. American Journal of Psychiatry. 1993;150:1139–1148. doi: 10.1176/ajp.150.8.1139. [DOI] [PubMed] [Google Scholar]
- Kendrick KM. Oxytocin, motherhood and bonding. Experimental Physiology. 2000;85 doi: 10.1111/j.1469-445x.2000.tb00014.x. Spec No, 111S–124S. [DOI] [PubMed] [Google Scholar]
- Kendrick KM, Levy F, Keverne EB. Changes in the sensory processing of olfactory signals induced by birth in sleep. Science. 1992;256:833–836. doi: 10.1126/science.1589766. [DOI] [PubMed] [Google Scholar]
- Killgore WD, Yurgelun-Todd DA. Ventromedial prefrontal activity correlates with depressed mood in adolescent children. Neuroreport. 2006;17:167–171. doi: 10.1097/01.wnr.0000198951.30939.73. [DOI] [PubMed] [Google Scholar]
- Kim-Cohen J, Moffitt TE, Taylor A, Pawlby SJ, Caspi A. Maternal depression and children’s antisocial behavior: Nature and nurture effects. Archives of General Psychiatry. 2005;62:173–181. doi: 10.1001/archpsyc.62.2.173. [DOI] [PubMed] [Google Scholar]
- Knutson B, Cooper JC. Functional magnetic resonance imaging of reward prediction. Current Opinion in Neurology. 2005;18:411–417. doi: 10.1097/01.wco.0000173463.24758.f6. [DOI] [PubMed] [Google Scholar]
- Komisaruk BR, Whipple B. Functional MRI of the brain during orgasm in women. Annual Review of Sex Research. 2005;16:62–86. [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 2001;24:97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- Kufahl PR, Li Z, Risinger RC, Rainey CJ, Wu G, Bloom AS, Li SJ. Neural responses to acute cocaine administration in the human brain detected by fMRI. NeuroImage. 2005;28:904–914. doi: 10.1016/j.neuroimage.2005.06.039. [DOI] [PubMed] [Google Scholar]
- LaBar KS, Cabeza R. Cognitive neuroscience of emotional memory. Nature Reviews. Neuroscience. 2006;7:54–64. doi: 10.1038/nrn1825. [DOI] [PubMed] [Google Scholar]
- Leckman JF, Feldman R, Swain JE, Eicher V, Thompson N, Mayes LC. Primary parental preoccupation: Circuits, genes, and the crucial role of the environment. Journal of Neural Transmission. 2004;111:753–771. doi: 10.1007/s00702-003-0067-x. [DOI] [PubMed] [Google Scholar]
- Leckman JF, Herman AE. Maternal behavior and developmental psychopathology. Biological Psychiatry. 2002;51:27–43. doi: 10.1016/s0006-3223(01)01277-x. [DOI] [PubMed] [Google Scholar]
- Leckman JF, Mayes LC, Feldman R, Evans DW, King RA, Cohen DJ. Early parental preoccupations and behaviors and their possible relationship to the symptoms of obsessivecompulsive disorder. Acta Psychiatrica Scandinavica. Supplementum. 1999;396:1–26. doi: 10.1111/j.1600-0447.1999.tb10951.x. [DOI] [PubMed] [Google Scholar]
- Lin HC, Xirasagar S. Institutional factors in cesarean delivery rates: Policy and research implications. Obstetrics and Gynecology. 2004;103:128–136. doi: 10.1097/01.AOG.0000102935.91389.53. [DOI] [PubMed] [Google Scholar]
- Lobel M, DeLuca RS. Psychosocial sequelae of cesarean delivery: Review and analysis of their causes and implications. Social Science and Medicine. 2007;64:2272–2284. doi: 10.1016/j.socscimed.2007.02.028. [DOI] [PubMed] [Google Scholar]
- Lorberbaum JP, Newman JD, Horwitz AR, Dubno JR, Lydiard RB, Hamner MB, Bohning DE, George MS. A potential role for thalamocingulate circuitry in human maternal behavior. Biological Psychiatry. 2002;51:431–445. doi: 10.1016/s0006-3223(01)01284-7. [DOI] [PubMed] [Google Scholar]
- Luthy DA, Malmgren JA, Zingheim RW, Leininger CJ. Physician contribution to a cesarean delivery risk model. American Journal of Obstetrics and Gynecoolgy. 2003;188:1579–1585. doi: 10.1067/mob.2003.389. [DOI] [PubMed] [Google Scholar]
- Martin JA, Hamilton BE, Sutton PD, Ventura SJ, Menacker F, Kirmeyer S. Births: Final data for 2004. National Vital Statistics Reports. 2006;55:1–101. [PubMed] [Google Scholar]
- Martin-Soelch C, Leenders KL, Chevalley AF, Missimer J, Kunig G, Magyar S, Mino A, Schultz W. Reward mechanisms in the brain and their role in dependence: Evidence from neurophysiological and neuroimaging studies. Brain Research. Brain Research Reviews. 2001;36:139–149. doi: 10.1016/s0165-0173(01)00089-3. [DOI] [PubMed] [Google Scholar]
- Mataix-Cols D, Wooderson S, Lawrence N, Brammer MJ, Speckens A, Phillips ML. Distinct neural correlates of washing, checking, and hoarding symptom dimensions in obsessive-compulsive disorder. Archives of General Psychiatry. 2004;61:564–576. doi: 10.1001/archpsyc.61.6.564. [DOI] [PubMed] [Google Scholar]
- Matthews TG, Crowley P, Chong A, McKenna P, McGarvey C, O’Regan M. Rising caesarean section rates: A cause for concern? BJOG. 2003;110:346–349. [PubMed] [Google Scholar]
- Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, Schwalb JM, Kennedy SH. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45:651–660. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- Mayes LC, Swain JE, Feldman R, Leckman JF. Primary parental preoccupation and the transition from adulthood to parenthood. Submitted. 2008 [Google Scholar]
- Mayes LC, Swain JE, Leckman JF. Parental attachment systems: Neural circuits, genes, and experiential contributions to parental engagement. Clinical Neuroscience Research. 2005;4:301–313. [Google Scholar]
- Morgan HD, Fleming AS, Stern JM. Somatosensory control of the onset and retention of maternal responsiveness in primiparous Sprague-Dawley rats. Physiology and Behavior. 1992;51:549–555. doi: 10.1016/0031-9384(92)90178-5. [DOI] [PubMed] [Google Scholar]
- Murphy DJ. Failure to progress in the second stage of labour. Current Opinion in Obstetrics and Gynecology. 2001;13:557–561. doi: 10.1097/00001703-200112000-00002. [DOI] [PubMed] [Google Scholar]
- Murphy DJ, Liebling RE, Patel R, Verity L, Swingler R. Cohort study of operative delivery in the second stage of labour and standard of obstetric care. BJOG. 2003;110:610–615. [PubMed] [Google Scholar]
- Murray L, Cooper PJ. The impact of postpartum depression on child development. In: Goodyer I, editor. Aetiological mechanisms in developmental psychopathology. Oxford University Press; Oxford: 2003. [Google Scholar]
- Newman JD. Neural circuits underlying crying and cry responding in mammals. Behavioural Brain Research. 2007;182:155–165. doi: 10.1016/j.bbr.2007.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Numan M. Hypothalamic neural circuits regulating maternal responsiveness toward infants. Behavioral and Cognitive Neuroscience Reviews. 2006;5:163–190. doi: 10.1177/1534582306288790. [DOI] [PubMed] [Google Scholar]
- Numan M. Motivational systems and the neural circuitry of maternal behavior in the rat. Developmental Psychobiology. 2007;49:12–21. doi: 10.1002/dev.20198. [DOI] [PubMed] [Google Scholar]
- Peterson RL. The neuroscience of investing: fMRI of the reward system. Brain Research Bulletin. 2005;67:391–397. doi: 10.1016/j.brainresbull.2005.06.015. [DOI] [PubMed] [Google Scholar]
- Phelps EA. Human emotion and memory: Interactions of the amygdala and hippocampal complex. Current Opinion in Neurobiology. 2004;14:198–202. doi: 10.1016/j.conb.2004.03.015. [DOI] [PubMed] [Google Scholar]
- Poindron P. Mechanisms of activation of maternal behaviour in mammals. Reproduction, Nutrition, Development. 2005;45:341–351. doi: 10.1051/rnd:2005025. [DOI] [PubMed] [Google Scholar]
- Porter RH, Duchamp G, Nowak R, Daels PF. Induction of maternal behavior in non-parturient adoptive mares. Physiology and Behavior. 2002;77:151–154. doi: 10.1016/s0031-9384(02)00819-3. [DOI] [PubMed] [Google Scholar]
- Posner MI, Rothbart MK. Attention, self-regulation and consciousness. Philosophical Transactions of the Royal Society London B: Biological Sciences. 1998;353:1915–1927. doi: 10.1098/rstb.1998.0344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romeyer A, Poindron P, Porter RH, Levy F, Orgeur P. Establishment of maternal bonding and its mediation by vaginocervical stimulation in goats. Physiology and Behavior. 1994;55:395–400. doi: 10.1016/0031-9384(94)90153-8. [DOI] [PubMed] [Google Scholar]
- Rosenberg TJ, Garbers S, Lipkind H, Chiasson MA. Maternal obesity and diabetes as risk factors for adverse pregnancy outcomes: Differences among 4 racial/ethnic groups. American Journal of Public Health. 2005;95:1545–1551. doi: 10.2105/AJPH.2005.065680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxe R. Uniquely human social cognition. Current Opinion in Neurobiology. 2006;16:235–239. doi: 10.1016/j.conb.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Schultz RT. Developmental deficits in social perception in autism: The role of the amygdala and fusiform face area. International Journal of Developmental Neuroscience. 2005;23:125–141. doi: 10.1016/j.ijdevneu.2004.12.012. [DOI] [PubMed] [Google Scholar]
- Schultz W. Multiple reward signals in the brain. Nature Reviews. Neuroscience. 2000;1:199–207. doi: 10.1038/35044563. [DOI] [PubMed] [Google Scholar]
- Soltis J. The signal functions of early infant crying. Behavioral and Brain Sciences. 2004;27:443–458. discussion 459–490. [PubMed] [Google Scholar]
- Sroufe LA, Carlson EA, Levy AK, Egeland B. Implications of attachment theory for developmental psychopathology. Development and Psychopathology. 1999;11:1–13. doi: 10.1017/s0954579499001923. [DOI] [PubMed] [Google Scholar]
- Swain JE, Lorberbaum JP. Imaging the human parental brain. In: Bridges R, editor. Neurobiology of the parental brain. Elsevier; San Diego, CA: 2008. pp. 83–100. [Google Scholar]
- Swain JE, Lorberbaum JP, Kose S, Strathearn L. Brain basis of early parent–infant interactions: Psychology, physiology, and in vivo functional neuroimaging studies. Journal of Child Psychology and Psychiatry. 2007;48:262–287. doi: 10.1111/j.1469-7610.2007.01731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain JE, Mayes LC, Leckman JF. Endogenous and exogenous opiates modulate the development of parent–infant attachment. Behavioral and Brain Sciences. 2005;28:364–365. [Google Scholar]
- Takahashi H, Matsuura M, Koeda M, Yahata N, Suhara T, Kato M, Okubo Y. Brain activations during judgments of positive self-conscious emotion and positive basic emotion: Pride and joy. Cerebral Cortex. 2008;18:898–903. doi: 10.1093/cercor/bhm120. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic altas of the human brain. Thieme Publishing Group; New York: 1988. [Google Scholar]
- Trujillo-Hernandez B, Rios-Silva M, Huerta M, Trujillo X, Vasquez C, Millan-Guerrero R. Frequency of, indications for and clinical epidemiological characteristics of first time cesarean section, compared with repeated cesarean section. Archives of Gynecology and Obstetrics. 2002;267:27–32. doi: 10.1007/s00404-001-0255-6. [DOI] [PubMed] [Google Scholar]
- Villar J, Valladares E, Wojdyla D, Zavaleta N, Carroli G, Velazco A, Shah A, Campodonico L, Bataglia V, Faundes A, Langer A, Narvaez A, Donner A, Romero M, Reynoso S, de Padua KS, Giordano D, Kublickas M, Acosta A. Caesarean delivery rates and pregnancy outcomes: The 2005 WHO global survey on maternal and perinatal health in Latin America. Lancet. 2006;367:1819–1829. doi: 10.1016/S0140-6736(06)68704-7. [DOI] [PubMed] [Google Scholar]
- Wiens S. Interoception in emotional experience. Current Opinion in Neurology. 2005;18:442–447. doi: 10.1097/01.wco.0000168079.92106.99. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Marrett S, Neelin P, Vandal AC, Friston KJ, Evans AC. A unified statistical approach for determining significant signals in images of cerebral activation. Human Brain Mapping. 1996;4:58–73. doi: 10.1002/(SICI)1097-0193(1996)4:1<58::AID-HBM4>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Xirasagar S, Lin HC, Liu TC. Do group practices have lower caesarean rates than solo practice obstetric clinics? Evidence from Taiwan. Health Policy Plan. 2006;21:319–325. doi: 10.1093/heapol/czl015. [DOI] [PubMed] [Google Scholar]
- Yeo JA, Keverne EB. The importance of vaginal-cervical stimulation for maternal behaviour in the rat. Physiology and Behavior. 1986;37:23–26. doi: 10.1016/0031-9384(86)90378-1. [DOI] [PubMed] [Google Scholar]