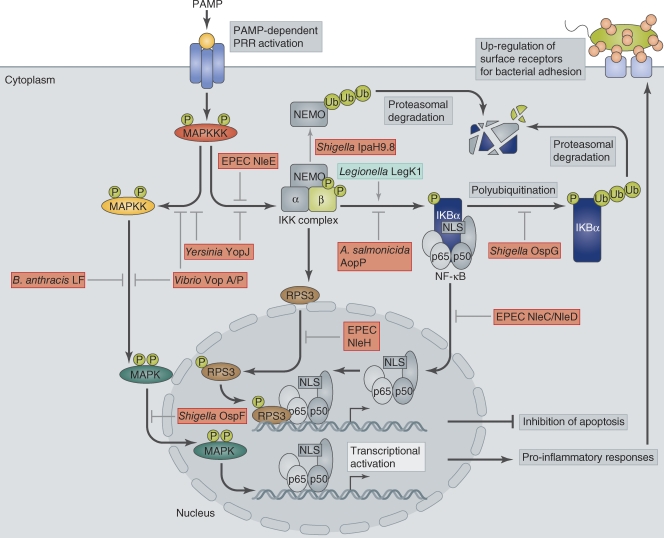
Figure 1.
Regulation of MAPK and NF-κB pathways and their manipulation by bacterial effectors. Bacterial infection triggers PAMP-dependent activation of PRRs at the host cell membrane. Receptor activation triggers MKKK phosphorylation, which in turn activates MAPK pathways and the NF-κB pathway via activation of the IKK complex. IKK phosphorylates IκB, which then becomes polyubiquitinated and removed by proteasomal degradation. Degradation of IκB exposes the NLS on NF-κB, which subsequently undergoes nuclear translocation and binds to κB sites in target promoters to stimulate transcription of genes required for pro-inflammatory responses, such as cytokines and chemokines, and genes involved in cell proliferation. Activation of NF-κB also leads to surface expression of receptors such as CEACAMs, which are hijacked by pathogens to promote their attachment to the host cell surface. Some κB site-containing promoters require RPS3 as a transcriptional co-factor in addition to NF-κB. RPS3 nuclear translocation is dependent on its phosphorylation by IKKβ. Bacterial pathogens manipulate both MAPK and NF-κB pathways either by acting as inhibitors (red boxes) or activators (green box) of individual components.