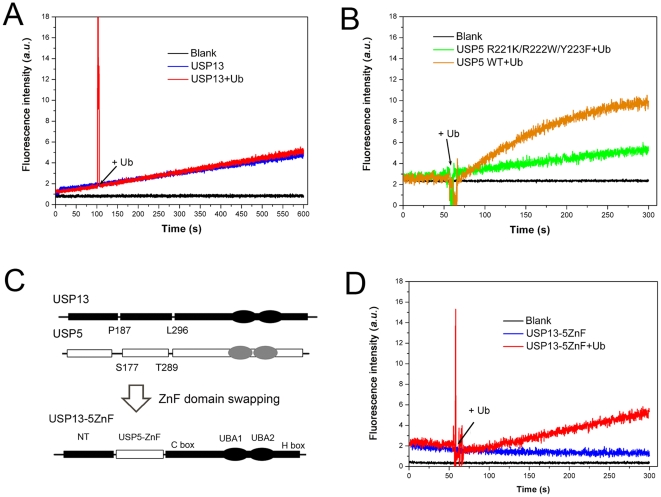
Figure 5. Gain of the catalytic activation of USP13 through ZnF-domain swapping from USP5.
A, Non-activating catalysis of USP13 by free Ub. Ub-AMC (250 nM) was incubated with GST-USP13 (1 µM) for 100 s, and then Ub (38 µM) was added into the reaction mixture. B, Activating catalysis of USP5 by free Ub. In comparison with wild-type USP5 that exhibits high Ub activation, the USP5 mutant (R221K/R222W/Y223F) loses the property of being activated by Ub. Ub-AMC (250 nM) was incubated with USP5 or its mutant (10 nM) for 50 s, and then Ub (100 nM) was added into the reaction mixture. C, Schematic diagram of the domain-swapped mutant of USP13. USP13-5ZnF denotes a chimera enzyme of USP13 with a substituted ZnF domain from USP5. D, USP13-5ZnF can be activated by free Ub. Ub-AMC (250 nM) was incubated with USP13-5ZnF (100 nM) for 50 s, and then Ub (1 µM) was added into the reaction mixture. All the reactions were monitored by fluorescence of released AMC at 460 nm.