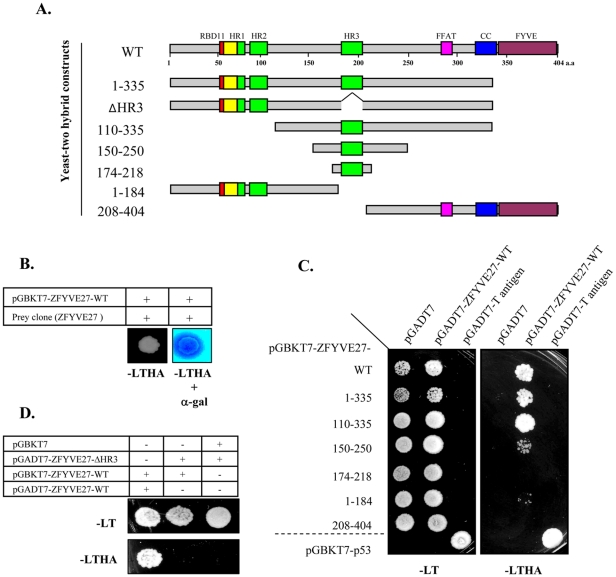
Figure 1. Yeast two-hybrid (Y2H) screen showing self-interaction of ZFYVE27.
(A) Schematic diagram showing the structural domains of ZFYVE27 and delineation of various deletion constructs of ZFYVE27. ZFYVE27 contains Rab11 binding domain RBD11 (red) at its N-terminus and FYVE domain (purple) at C-terminus. The three hydrophobic regions (HR) are depicted in green and the overlapping region of HR1 region with the RBD11 motif is highlighted in yellow. The FFAT motif (pink) and the coiled-coil region (blue) mediate interaction with VAP-A protein. The generated deletion constructs for Y2H analysis are depicted thereof. (B) Activation of GAL4 reporter genes by interaction of ZFYVE27 with the prey clone (ZFYVE27) in the Y2H screen. A robust growth of yeast strain AH109 was observed on the nutritional selection medium -LTHA (lacking leucine, tryptophan, histidine and adenine) and also was positive for the α-galactosidase (α-gal) activity. (C) Determination of the core interaction region of ZFYVE27, which mediate self-interaction by direct-Y2H. The indicated deletion constructs of ZFYVE27 were fused with DNA binding domain of GAL4 and evaluated for their ability to interact with full-length ZFYVE27 fused to activation domain of GAL4 in Y2H experiments. The interaction between p53 and T-antigen was used as a positive control in the Y2H assay. (D) Evaluation of ZFYVE27-ΔHR3 (deletion of HR3 in ZFYVE271-335 construct) interaction with full-length ZFYVE27.