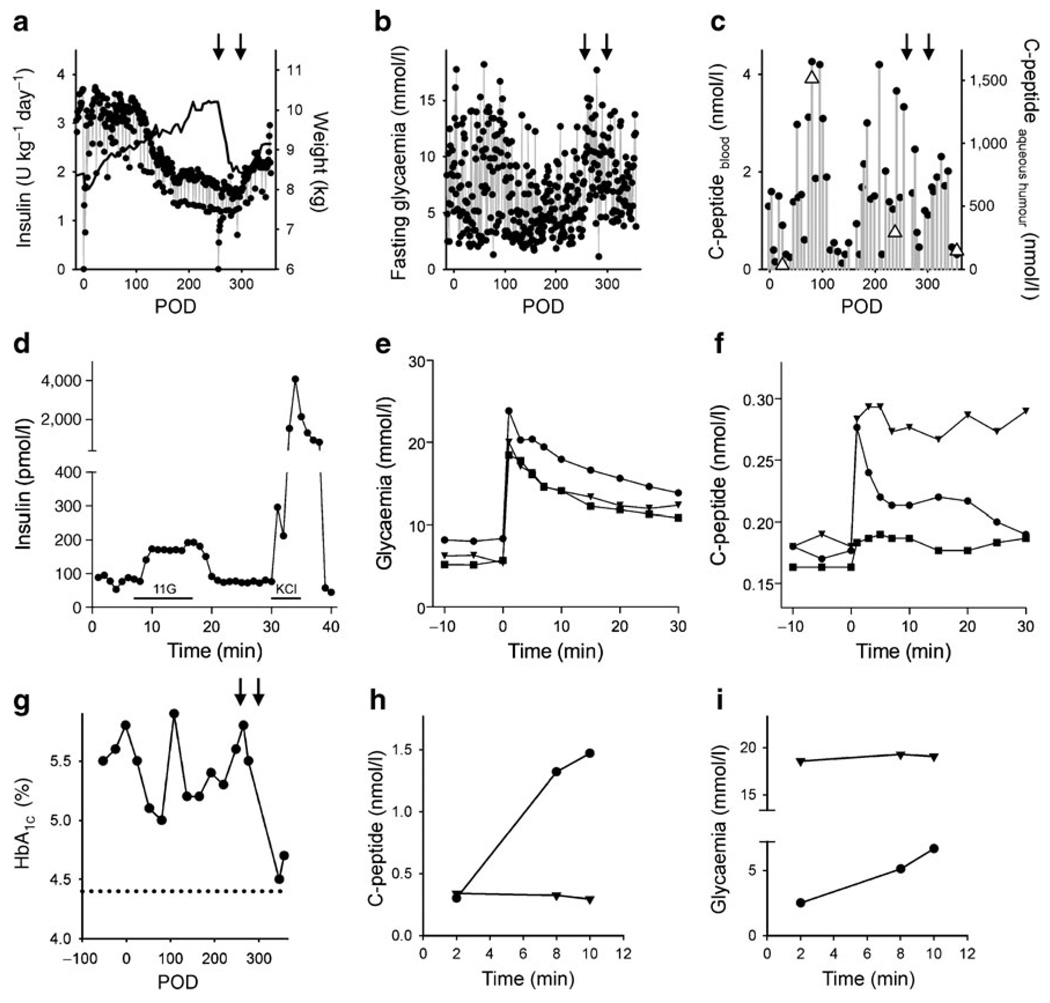
Fig. 2.
Pancreatic islet transplantation into the anterior chamber of the eye improved glycaemic control in a baboon model for diabetes. a Daily insulin requirements before and after islet transplantation. The baboon gained weight after transplantation (black line) until it was pancreatectomised (left-hand arrow in a–c, and g). Weight gain was seen again after a second islet transplantation (right-hand arrow in a–c, and g). b Fluctuations in fasting blood glucose decreased after transplantation, increased after pancreatectomy, and stabilised again after a second islet transplantation. c Plasma C-peptide levels increased after islet transplantation. C-peptide levels were much higher in the aqueous humour of the eye (triangles), but co-varied with plasma C-peptide levels. d Perifusion studies performed on isolated islets before transplantation showed insulin responses to high glucose (11 mmol/l; 11G) and KCl depolarisation (25 mmol/l). Bars under trace indicate stimulus applications. e, f Glucose excursions (e) and C-peptide blood levels (f) during IVGTTs performed at POD 54 (squares), POD 175 (triangles), and POD 271 (circles). g HbA1c levels decreased after islet transplantation and, after the second islet transplantation, almost reached levels measured in the same baboon before diabetes was induced (dotted line). h, i An injection of glucagon to stimulate insulin secretion (glucagon challenge) showed that the insulin secretory response was abolished after removal of the intraocular islet grafts. h Plasma C-peptide levels increased during a glucagon challenge before (circles) but not after removal of the transplanted eye (triangles) and glycaemia did not change with the glucagon challenge after removal of the transplanted eye, indicating that the baboon had lost responsiveness to glucagon (i)